Page 618 - Handbook of Battery Materials
P. 618
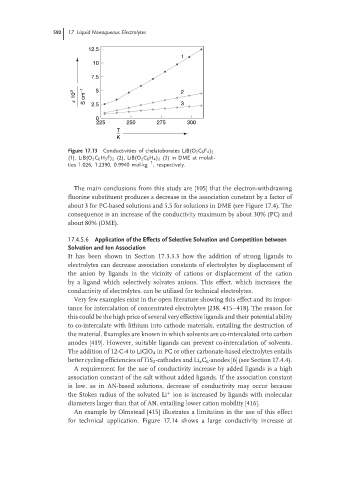
592 17 Liquid Nonaqueous Electrolytes
12.5
1
10
7.5
k 10 3 S cm −1 2.5 5 2
3
0
225 250 275 300
T
K
Figure 17.13 Conductivities of chelatoborates LiB(O 2 C 6 F 4 ) 2
(1), LiB(O 2 C 6 H 3 F) 2 (2), LiB(O 2 C 6 H 4 ) 2 (3) in DME at molali-
−1
ties 1.026, 1.2390, 0.9940 mol·kg , respectively.
The main conclusions from this study are [105] that the electron-withdrawing
fluorine substituent produces a decrease in the association constant by a factor of
about 3 for PC-based solutions and 5.5 for solutions in DME (see Figure 17.4). The
consequence is an increase of the conductivity maximum by about 30% (PC) and
about 80% (DME).
17.4.5.6 Application of the Effects of Selective Solvation and Competition between
Solvation and Ion Association
It has been shown in Section 17.3.3.3 how the addition of strong ligands to
electrolytes can decrease association constants of electrolytes by displacement of
the anion by ligands in the vicinity of cations or displacement of the cation
by a ligand which selectively solvates anions. This effect. which increases the
conductivity of electrolytes. can be utilized for technical electrolytes.
Very few examples exist in the open literature showing this effect and its impor-
tance for intercalation of concentrated electrolytes [238, 415–418]. The reason for
this could be the high price of several very effective ligands and their potential ability
to co-intercalate with lithium into cathode materials, entailing the destruction of
the material. Examples are known in which solvents are co-intercalated into carbon
anodes [419]. However, suitable ligands can prevent co-intercalation of solvents.
The addition of 12-C-4 to LiClO 4 in PC or other carbonate-based electrolytes entails
better cycling efficiencies of TiS 2 -cathodes and Li x C 6 -anodes [6] (see Section 17.4.4).
A requirement for the use of conductivity increase by added ligands is a high
association constant of the salt without added ligands. If the association constant
is low, as in AN-based solutions, decrease of conductivity may occur because
+
the Stokes radius of the solvated Li ion is increased by ligands with molecular
diameters larger than that of AN, entailing lower cation mobility [416].
An example by Olmstead [415] illustrates a limitation in the use of this effect
for technical application. Figure 17.14 shows a large conductivity increase at