Page 617 - Handbook of Battery Materials
P. 617
17.4 Bulk Properties 591
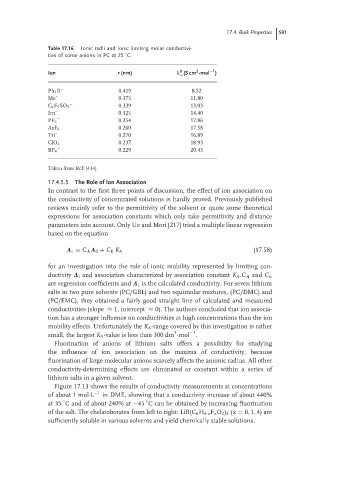
Table 17.16 Ionic radii and ionic limiting molar conductivi-
◦
ties of some anions in PC at 25 C.
−1
2
0
Ion r (nm) λ (S cm ·mol )
−
Ph 4 B − 0.419 8.52
Me – 0.375 11.80
− 0.339 13.03
C 4 F 9 SO 3
Im – 0.325 14.40
− 0.254 17.86
PF 6
− 0.260 17.58
AsF 6
Tri – 0.270 16.89
− 0.237 18.93
ClO 4
−
BF 4 0.229 20.43
Taken from Ref. [414].
17.4.5.5 The Role of Ion Association
In contrast to the first three points of discussion, the effect of ion association on
the conductivity of concentrated solutions is hardly proved. Previously published
reviews mainly refer to the permittivity of the solvent or quote some theoretical
expressions for association constants which only take permittivity and distance
parameters into account. Only Ue and Mori [217] tried a multiple linear regression
based on the equation
Λ c = C Λ Λ 0 + C K K A (17.58)
for an investigation into the role of ionic mobility represented by limiting con-
ductivity Λ i and association characterized by association constant K A .C Λ and C K
are regression coefficients and Λ c is the calculated conductivity. For seven lithium
salts in two pure solvents (PC/GBL) and two equimolar mixtures, (PC/DMC) and
(PC/EMC), they obtained a fairly good straight line of calculated and measured
conductivities (slope ≈ 1, intercept ≈ 0). The authors concluded that ion associa-
tion has a stronger influence on conductivities at high concentrations than the ion
mobility effects. Unfortunately the K A -range covered by this investigation is rather
3
−1
small, the largest K A -value is less than 300 dm ·mol .
Fluorination of anions of lithium salts offers a possibility for studying
the influence of ion association on the maxima of conductivity, because
fluorination of large molecular anions scarcely affects the anionic radius. All other
conductivity-determining effects are eliminated or constant within a series of
lithium salts in a given solvent.
Figure 17.13 shows the results of conductivity measurements at concentrations
of about 1 mol·L −1 in DME, showing that a conductivity increase of about 440%
◦
◦
at 35 C and of about 240% at −45 C can be obtained by increasing fluorination
of the salt. The chelatoborates from left to right: LiB(C 6 H 4-x F x O 2 ) 2 (x = 0, 1, 4) are
sufficiently soluble in various solvents and yield chemically stable solutions.