Page 38 - Handbook of Energy Engineering Calculations
P. 38
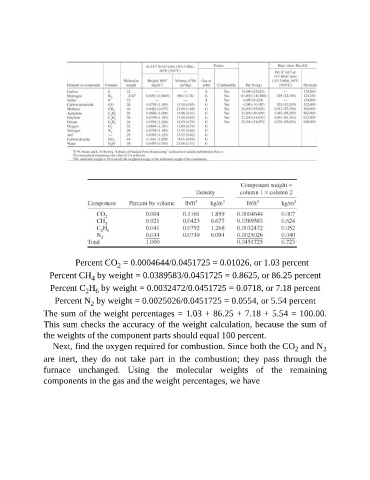
Percent CO = 0.0004644/0.0451725 = 0.01026, or 1.03 percent
2
Percent CH by weight = 0.0389583/0.0451725 = 0.8625, or 86.25 percent
4
Percent C H by weight = 0.0032472/0.0451725 = 0.0718, or 7.18 percent
2 6
Percent N by weight = 0.0025026/0.0451725 = 0.0554, or 5.54 percent
2
The sum of the weight percentages = 1.03 + 86.25 + 7.18 + 5.54 = 100.00.
This sum checks the accuracy of the weight calculation, because the sum of
the weights of the component parts should equal 100 percent.
Next, find the oxygen required for combustion. Since both the CO and N 2
2
are inert, they do not take part in the combustion; they pass through the
furnace unchanged. Using the molecular weights of the remaining
components in the gas and the weight percentages, we have