Page 39 - Handbook of Energy Engineering Calculations
P. 39
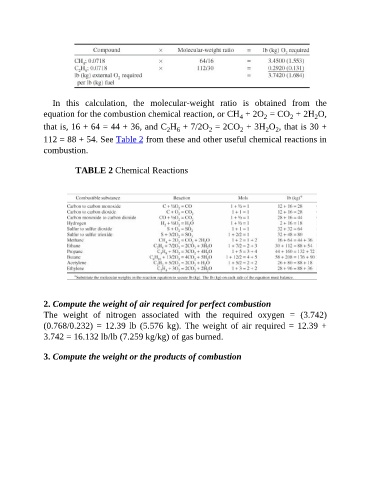
In this calculation, the molecular-weight ratio is obtained from the
equation for the combustion chemical reaction, or CH + 2O = CO + 2H O,
2
2
4
2
that is, 16 + 64 = 44 + 36, and C H + 7/2O = 2CO + 3H O , that is 30 +
2
2
2 6
2 2
112 = 88 + 54. See Table 2 from these and other useful chemical reactions in
combustion.
TABLE 2 Chemical Reactions
2. Compute the weight of air required for perfect combustion
The weight of nitrogen associated with the required oxygen = (3.742)
(0.768/0.232) = 12.39 lb (5.576 kg). The weight of air required = 12.39 +
3.742 = 16.132 lb/lb (7.259 kg/kg) of gas burned.
3. Compute the weight or the products of combustion