Page 35 - Handbook of Energy Engineering Calculations
P. 35
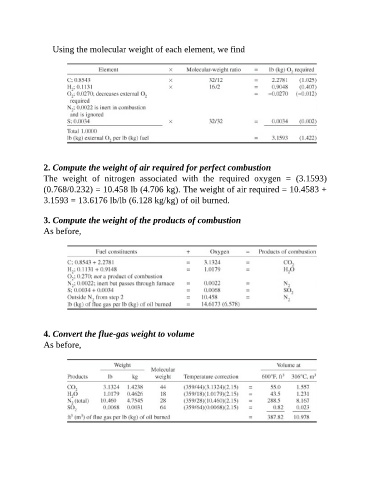
Using the molecular weight of each element, we find
2. Compute the weight of air required for perfect combustion
The weight of nitrogen associated with the required oxygen = (3.1593)
(0.768/0.232) = 10.458 lb (4.706 kg). The weight of air required = 10.4583 +
3.1593 = 13.6176 lb/lb (6.128 kg/kg) of oil burned.
3. Compute the weight of the products of combustion
As before,
4. Convert the flue-gas weight to volume
As before,