Page 457 - Handbook of Properties of Textile and Technical Fibres
P. 457
430 Handbook of Properties of Textile and Technical Fibres
was in the narrow range of 130e150 mN, elongation varied between 28% and 68%,
and flexing resistance (expressed in terms of the number of flex to break) was in the
range 1220e2200 (Militký et al., 1991). In comparison with other modification
methods used to obtain similar effects the changing of draw-setting conditions does
not involve the deterioration of the mechanical and physical properties of the fibers.
13.2.2.1 Chemical modification
The simplest chemical modification is the “unwanted” modification with DEG, which
is produced by the effect of side reactions during the production of PET fibers.
This modification only increases the length and mobility of aliphatic chains. This leads
to the formation of different configurations composed of trans-and gauche-
conformations of individual groups in PET chains. In some patents it has been sug-
gested, however, that it is possible to enhance dyeability by adding DEG intentionally
during polycondensation. The effect of DEG content on the dyeability of PET fibers
was studied in Militký et al. (1980b).
If ethylene glycol is replaced by higher diols, a higher number of configurations will
be formed in the chains. For example, in case of PBT the glycol residue will exhibit a
gauche-trans-gauche-conformation (Bereton et al., 1978). With the growing number
of methylene groups the chains will contain “shortened” spatial conformations,
composed of trans- and gauche-conformers (called a-forms) and “extended” planar
conformations, formed from trans-conformers only (called b-conformations). During
deformation the a-forms will be elastically changed into extended b-forms. The copol-
ymers based on PET always contain a relatively high number of benzene nuclei in the
para-position, which makes the chains more rigid. Likewise the polar forces, generated
by ester groups, hinder the mobility of chains at low temperatures (Mattes and
Roskow, 1966).
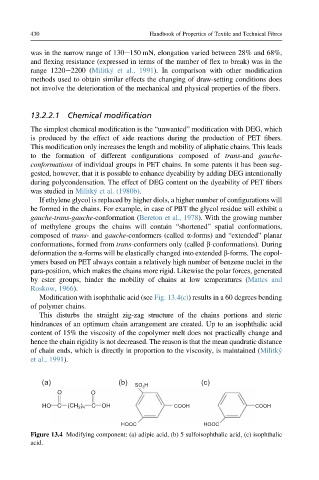
Modification with isophthalic acid (see Fig. 13.4(c)) results in a 60 degrees bending
of polymer chains.
This disturbs the straight zig-zag structure of the chains portions and steric
hindrances of an optimum chain arrangement are created. Up to an isophthalic acid
content of 15% the viscosity of the copolymer melt does not practically change and
hence the chain rigidity is not decreased. The reason is that the mean quadratic distance
of chain ends, which is directly in proportion to the viscosity, is maintained (Militký
et al., 1991).
(a) (b) (c)
SO H
3
O O
HO C (CH ) COH COOH COOH
2 4
HOOC HOOC
Figure 13.4 Modifying component: (a) adipic acid, (b) 5 sulfoisophthalic acid, (c) isophthalic
acid.