Page 460 - Handbook of Properties of Textile and Technical Fibres
P. 460
Tensile failure of polyester fibers 433
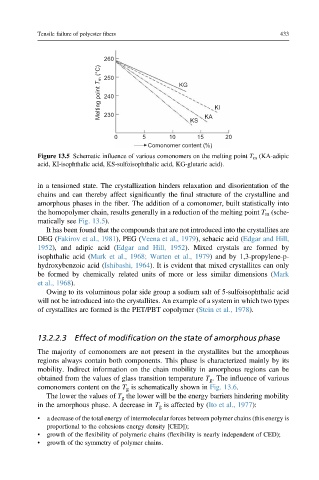
260
Melting point T m (°C) 250 KG
240
230
KS KA KI
0 5 10 15 20
Comonomer content (%)
Figure 13.5 Schematic influence of various comonomers on the melting point T m (KA-adipic
acid, KI-isophthalic acid, KS-sulfoisophthalic acid, KG-glutaric acid).
in a tensioned state. The crystallization hinders relaxation and disorientation of the
chains and can thereby affect significantly the final structure of the crystalline and
amorphous phases in the fiber. The addition of a comonomer, built statistically into
the homopolymer chain, results generally in a reduction of the melting point T m (sche-
matically see Fig. 13.5).
It has been found that the compounds that are not introduced into the crystallites are
DEG (Fakirov et al., 1981), PEG (Veena et al., 1979), sebacic acid (Edgar and Hill,
1952), and adipic acid (Edgar and Hill, 1952). Mixed crystals are formed by
isophthalic acid (Mark et al., 1968; Warten et al., 1979) and by 1,3-propylene-p-
hydroxybenzoic acid (Ishibashi, 1964). It is evident that mixed crystallites can only
be formed by chemically related units of more or less similar dimensions (Mark
et al., 1968).
Owing to its voluminous polar side group a sodium salt of 5-sulfoisophthalic acid
will not be introduced into the crystallites. An example of a system in which two types
of crystallites are formed is the PET/PBT copolymer (Stein et al., 1978).
13.2.2.3 Effect of modification on the state of amorphous phase
The majority of comonomers are not present in the crystallites but the amorphous
regions always contain both components. This phase is characterized mainly by its
mobility. Indirect information on the chain mobility in amorphous regions can be
obtained from the values of glass transition temperature T g . The influence of various
comonomers content on the T g is schematically shown in Fig. 13.6.
The lower the values of T g the lower will be the energy barriers hindering mobility
in the amorphous phase. A decrease in T g is affected by (Ito et al., 1977):
• a decrease of the total energy of intermolecular forces between polymer chains (this energy is
proportional to the cohesions energy density [CED]);
• growth of the flexibility of polymeric chains (flexibility is nearly independent of CED);
• growth of the symmetry of polymer chains.