Page 127 - Handbook of Surface Improvement and Modification
P. 127
122 Easy Surface Cleaning and Stain Inhibition
Considering difficulties with
cleaning the cleaners and their dis-
posal, the interest in self-cleaning
and stain-resistant materials
makes sense. Although, it is also
not free of flaws as the next
reported article shows. The degra-
dation of stain-resistant coating
materials leads to the release of
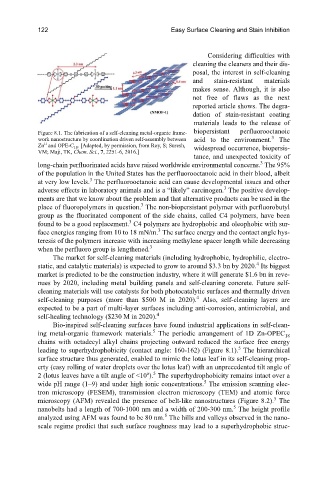
Figure 8.1. The fabrication of a self-cleaning metal-organic frame- biopersistant perfluorooctanoic
3
work nanostructure by coordination driven self-assembly between acid to the environment. The
II
Zn and OPE-C 18 . [Adapted, by permission, from Roy, S; Suresh, widespread occurrence, biopersis-
VM; Maji, TK, Chem. Sci., 7, 2251-6, 2016.]
tance, and unexpected toxicity of
3
long-chain perfluorinated acids have raised worldwide environmental concerns. The 95%
of the population in the United States has the perfluorooctanoic acid in their blood, albeit
3
at very low levels. The perfluorooctanoic acid can cause developmental issues and other
3
adverse effects in laboratory animals and is a “likely” carcinogen. The positive develop-
ments are that we know about the problem and that alternative products can be used in the
3
place of fluoropolymers in question. The non-biopersistant polymer with perfluorobutyl
group as the fluorinated component of the side chains, called C4 polymers, have been
3
found to be a good replacement. C4 polymers are hydrophobic and oleophobic with sur-
3
face energies ranging from 10 to 18 mN/m. The surface energy and the contact angle hys-
teresis of the polymers increase with increasing methylene spacer length while decreasing
3
when the perfluoro group is lengthened.
The market for self-cleaning materials (including hydrophobic, hydrophilic, electro-
4
static, and catalytic materials) is expected to grow to around $3.3 bn by 2020. Its biggest
market is predicted to be the construction industry, where it will generate $1.6 bn in reve-
nues by 2020, including metal building panels and self-cleaning concrete. Future self-
cleaning materials will use catalysts for both photocatalytic surfaces and thermally driven
4
self-cleaning purposes (more than $500 M in 2020). Also, self-cleaning layers are
expected to be a part of multi-layer surfaces including anti-corrosion, antimicrobial, and
4
self-healing technology ($230 M in 2020).
Bio-inspired self-cleaning surfaces have found industrial applications in self-clean-
5
ing metal-organic framework materials. The periodic arrangement of 1D Zn-OPEC 18
chains with octadecyl alkyl chains projecting outward reduced the surface free energy
5
leading to superhydrophobicity (contact angle: 160-162) (Figure 8.1). The hierarchical
surface structure thus generated, enabled to mimic the lotus leaf in its self-cleaning prop-
erty (easy rolling of water droplets over the lotus leaf) with an unprecedented tilt angle of
o 5
2 (lotus leaves have a tilt angle of <10 ). The superhydrophobicity remains intact over a
5
wide pH range (1–9) and under high ionic concentrations. The emission scanning elec-
tron microscopy (FESEM), transmission electron microscopy (TEM) and atomic force
5
microscopy (AFM) revealed the presence of belt-like nanostructures (Figure 8.2). The
5
nanobelts had a length of 700-1000 nm and a width of 200-300 nm. The height profile
5
analyzed using AFM was found to be 80 nm. The hills and valleys observed in the nano-
scale regime predict that such surface roughness may lead to a superhydrophobic struc-