Page 100 - High Power Laser Handbook
P. 100
70 G a s , C h e m i c a l , a n d F r e e - E l e c t r o n L a s e r s Chemical Lasers 71
Basic hydrogen
peroxide droplets
Chlorine Electronically
gas excited oxygen
1
O ( ∆)
2
I*
Iodine molecules are
dissociated and excited by
Salt byproduct 1
O ( ∆)
2
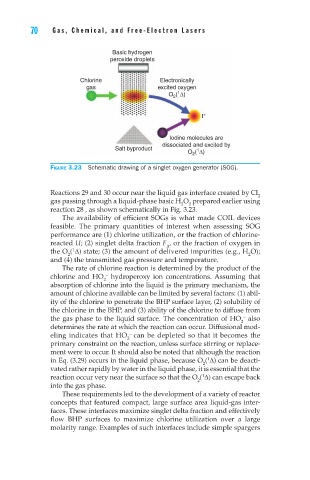
Figure 3.23 Schematic drawing of a singlet oxygen generator (SOG).
Reactions 29 and 30 occur near the liquid gas interface created by Cl
2
gas passing through a liquid-phase basic H O prepared earlier using
2
2
reaction 28 , as shown schematically in Fig. 3.23.
The availability of efficient SOGs is what made COIL devices
feasible. The primary quantities of interest when assessing SOG
performance are (1) chlorine utilization, or the fraction of chlorine-
reacted U; (2) singlet delta fraction F , or the fraction of oxygen in
∆
1
the O ( ∆) state; (3) the amount of delivered impurities (e.g., H O);
2
2
and (4) the transmitted gas pressure and temperature.
The rate of chlorine reaction is determined by the product of the
–
chlorine and HO hydroperoxy ion concentrations. Assuming that
2
absorption of chlorine into the liquid is the primary mechanism, the
amount of chlorine available can be limited by several factors: (1) abil-
ity of the chlorine to penetrate the BHP surface layer, (2) solubility of
the chlorine in the BHP, and (3) ability of the chlorine to diffuse from
the gas phase to the liquid surface. The concentration of HO also
–
2
determines the rate at which the reaction can occur. Diffusional mod-
eling indicates that HO can be depleted so that it becomes the
–
2
primary constraint on the reaction, unless surface stirring or replace-
ment were to occur. It should also be noted that although the reaction
in Eq. (3.29) occurs in the liquid phase, because O ( ∆) can be deacti-
1
2
vated rather rapidly by water in the liquid phase, it is essential that the
1
reaction occur very near the surface so that the O ( ∆) can escape back
2
into the gas phase.
These requirements led to the development of a variety of reactor
concepts that featured compact, large surface area liquid-gas inter-
faces. These interfaces maximize singlet delta fraction and effectively
flow BHP surfaces to maximize chlorine utilization over a large
molarity range. Examples of such interfaces include simple spargers