Page 96 - High Power Laser Handbook
P. 96
66 G a s , C h e m i c a l , a n d F r e e - E l e c t r o n L a s e r s Chemical Lasers 67
BHP Optics
f
O 2 ( D)
Chlorine Singlet
(Cl 2 + He) oxyzen
NH 3 generator
Discharges Main heat Iodine
directly outside exchanger (I 2 & He) HOT
aircraft ammonia (NH 3 ) BHP+ Gain generator I*, O 2 ( D)
f
during flight H 2 O,
salt,
heat
Diffuser
Hydrogen peroxide Turbo-
(H 2 O 2 ) generator Gas pump Hydrogen peroxide
Optics
(H 2 O 2 )
Pressure recovery system
1.315 m
output beam
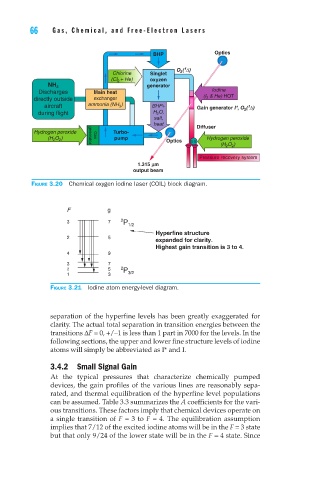
Figure 3.20 Chemical oxygen iodine laser (COIL) block diagram.
F g
3 7 2 P 1/2
Hyperfine structure
2 5 expanded for clarity.
Highest gain transition is 3 to 4.
4 9
3 7
2 5 2 P
1 3 3/2
Figure 3.21 Iodine atom energy-level diagram.
separation of the hyperfine levels has been greatly exaggerated for
clarity. The actual total separation in transition energies between the
transitions ∆F = 0, +/–1 is less than 1 part in 7000 for the levels. In the
following sections, the upper and lower fine structure levels of iodine
atoms will simply be abbreviated as I* and I.
3.4.2 Small Signal Gain
At the typical pressures that characterize chemically pumped
devices, the gain profiles of the various lines are reasonably sepa-
rated, and thermal equilibration of the hyperfine level populations
can be assumed. Table 3.3 summarizes the A coefficients for the vari-
ous transitions. These factors imply that chemical devices operate on
a single transition of F = 3 to F = 4. The equilibration assumption
implies that 7/12 of the excited iodine atoms will be in the F = 3 state
but that only 9/24 of the lower state will be in the F = 4 state. Since