Page 32 - High Power Laser Handbook
P. 32
4 G a s , C h e m i c a l , a n d F r e e - E l e c t r o n L a s e r s Carbon Dioxide Lasers 5
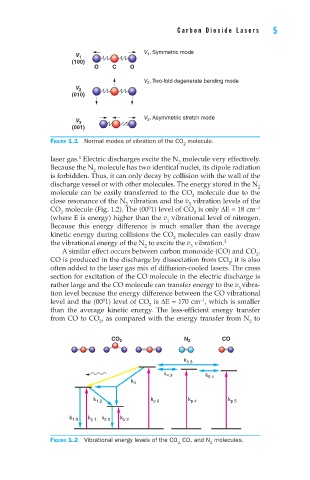
V 1 , Symmetric mode
V 1
(100)
O C O
V 2 , Two-fold degenerate bending mode
V 2
(010)
V 3 , Asymmetric stretch mode
V 3
(001)
Figure 1.1 Normal modes of vibration of the CO molecule.
2
1
laser gas. Electric discharges excite the N molecule very effectively.
2
Because the N molecule has two identical nuclei, its dipole radiation
2
is forbidden. Thus, it can only decay by collision with the wall of the
discharge vessel or with other molecules. The energy stored in the N
2
molecule can be easily transferred to the CO molecule due to the
2
close resonance of the N vibration and the v vibration levels of the
3
2
CO molecule (Fig. 1.2). The (00 1) level of CO is only ∆E = 18 cm –1
0
2
2
(where E is energy) higher than the v vibrational level of nitrogen.
1
Because this energy difference is much smaller than the average
kinetic energy during collisions the CO molecules can easily draw
2
the vibrational energy of the N to excite the v vibration. 2
2
3
A similar effect occurs between carbon monoxide (CO) and CO .
2
CO is produced in the discharge by dissociation from CO ; it is also
2
often added to the laser gas mix of diffusion-cooled lasers. The cross
section for excitation of the CO molecule in the electric discharge is
rather large and the CO molecule can transfer energy to the v vibra-
3
tion level because the energy difference between the CO vibrational
level and the (00 1) level of CO is ∆E = 170 cm , which is smaller
0
–1
2
than the average kinetic energy. The less-efficient energy transfer
from CO to CO , as compared with the energy transfer from N to
2 2
CO 2 N 2 CO
k 5 3
k 4 3
k 5 4
k 3
k 1 2 k p 8 k p 4 k p 5
k 1 0 k p 1 k 2 0 k p 2
Figure 1.2 Vibrational energy levels of the CO CO, and N molecules.
2, 2