Page 143 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 143
120 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
For intermediate temperature SOFCs, a composite cathode consisting of
strontium-doped lanthanum manganite (LSM) and YSZ has shown good
performance [ 101. For use with ceria-based electrolytes [ll], a (La,Sr)(Co,Fe)03
(LSCF)-based cathode has been developed [ 121.
In this chapter, the physical and physicochemical properties of perovskites
which are important in understanding electrochemical performance are given in
detail, emphasising the effect of valence stability on oxygen nonstoichiometry,
crystal structure, electrical conductivity, thermal expansion and chemical
reactivity with YSZ electrolyte. Then, practical aspects of the lanthanum
manganite cathodes are described with a focus on how to avoid La2Zr207
formation. Cathodes for the intermediate temperature SOFCs are discussed in
relation to the conventional LaMn03-based cathodes. Compatibility of cathode
materials with oxide ceramic interconnects and metallic interconnects is also
examined with emphasis on Cr poisoning. Finally, the fabrication methods for
cathodes are briefly described.
5.2 Physical and Physicochemical Properties of Perovskite
Cathode Materials
5.2.1 Lattice Structure, Oxygen Nonstoichiometry, and Valence Stability
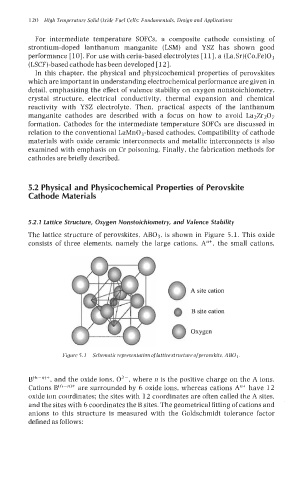
The lattice structure of perovskites, AB03, is shown in Figure 5.1. This oxide
consists of three elements, namely the large cations, A"+, the small cations,
A site cation
B site cation
Oxygen
Figure 5.1 Schematic representation oflattice structure ofperovskite, AB0 3.
B(6-")+, and the oxide ions, 02-, where n is the positive charge on the A ions.
Cations B(6-")+ are surrounded by 6 oxide ions, whereas cations A"+ have 12
oxide ion coordinates: the sites with 12 coordinates are often called the A sites,
and the sites with 6 coordinates the B sites. The geometrical fitting of cations and
anions to this structure is measured with the Goldschmidt tolerance factor
defined as follows: