Page 147 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 147
124 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
I
5.8
5.6 _. . . .
_. . .
.
-'
5.4
-. . . .
'E 5.2
g5
_. . . .
-
v
2 4.8
4.6
4.4
4.2
4
0 o.ooo5 0.001 0.0015 0.002
T'K'
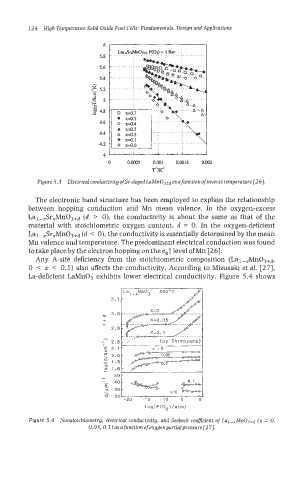
Figure 5.3 Electrical conductivity of Sr-doped LaMnO3+d as a function of inverse temperature 1261.
The electronic band structure has been employed to explain the relationship
between hopping conduction and Mn mean valence. In the oxygen-excess
Lal-,Sr,Mn03+d (d > 0), the conductivity is about the same as that of the
material with stoichiometric oxygen content, d = 0. In the oxygen-deficient
Lal-,Sr,Mn03+d (d < 0), the conductivity is essentially determined by the mean
Mn valence and temperature. The predominant electrical conduction was found
to take place by the electron hopping on the egT level of Mn [26].
Any A-site deficiency from the stoichiometric composition (Lal-,Mn03+d,
0 < x < 0.1) also affects the conductivity. According to Mizusaki et aI. 1271,
La-deficient LaMn03 exhibits lower electrical conductivity. Figure 5.4 shows
3.1
TI 3.0
+
0
2.9
-
- 2.8
5 2.1
2 2.0
2 1.9
or
0 1.8
-
.-.. 50
.
0
2o -20 -15 -10 -5 C
Iog(P(O2)/atm)
=
Figure 5.4 Nonstoichiometry, electrical conductivity, and Seebeck coefficient of Lu~-,M~O~+~ 0,
(x
0.05.0.1) asafunctionofoxygenpartialpressure[27].