Page 144 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 144
Cathodes 121
where rA, rB. and ro are the effective ionic radii of A, B, and 0 ions, respectively.
Usually, this factor is evaluated from Shannon's ionic radii [ 131 for respective
coordination numbers. When the tolerance factor is near unity, the structure is
the ideal cubic one. In other cases, orthorhombic or rhombohedral distortions
appear.
Undoped stoichiometric LaMnOs shows orthorhombic structure at 2 5°C and
rhombohedral above 600°C [14]. The crystallographic transformation
temperature strongly depends on the oxygen stoichiometry, and hence on the
mean manganese valence. With increasing oxygen content, the transformation
temperature decreases rapidly. Substitution of La with lower valence cations
(such as Sr2+ and Ca2+) or A-site deficiency increases the concentration of
Mn4' in the LaMn03 lattice. This eventually decreases the transformation
temperature. Although the cubic structure does not appear in pure LaMn03, it
appears around 1000°C in (Lao.7Sro.3)Mn03.
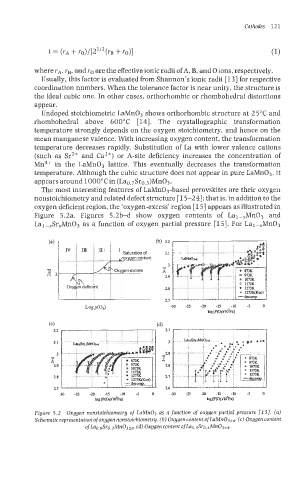
The most interesting features of LaMn03-based perovslrites are their oxygen
nonstoichiometry and related defect structure [15-241; that is, in addition to the
oxygen deficient region, the 'oxygen-excess' region [ 151 appears as illustrated in
Figure 5.2a. Figures 5.2b-d show oxygen contents of Lal-,Mn03 and
Lal-xSrxMn03 as a function of oxygen partial pressure [15]. For Lal-,MnO3
(b) 3.2
3. I
3
.a
... ................... ........... A
2.9
i
2.8
27
-30 -25 -20 -15 -10 -5 0
bg [P(OWO'P~I
3. I
Figure 5.2 Oxygen nonstoichiometry of LaMn03 as a function of oxygen partial pressure 1151. (a)
(c)
Schematic representation of oxygen nonstoichiometry. (b) Oxygen content of LUM~O~?~. Oxygen content
0fLa~.~Sr~.~Mn0~+~.
(a) Oxygen content 0fLa~.~Sr~.~Mn0~+~.