Page 150 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 150
Cathodes 127
temperature range for various compositions of perovskite materials. Since these
materials are electronically conducting, there are many mobile electrons. On
oxygen incorporation, therefore, the concentration of oxide ion vacancies plays
an important role both for surface oxygen exchange and oxide ion diffusion.
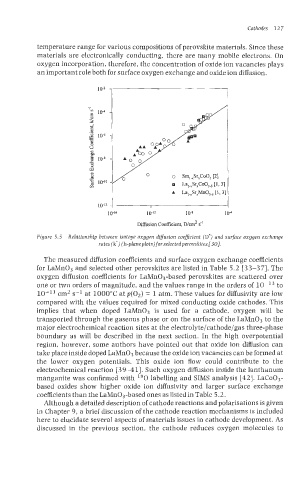
10-16 10-12 108 10-4
Diffusion Coefficient, D/cm' s-'
Figure 5.5 Relationship between isotope oxggen diflmion coeflcient (D') and surface oxggen exchange
rates (k-) (h-planeplots) for selectedperovskites [301.
The measured diffusion coefficients and surface oxygen exchange coefficients
for LaMn03 and selected other perovslcites are listed in Table 5.2 [33-3 71. The
oxygen diffusion coefficients for LaMn03-based perovskites are scattered over
one or two orders of magnitude, and the values range in the orders of to
10-l' cm2 s-l at 1000°C at p(Oz) = 1 atm. These values for diffusivity are low
compared with the values required for mixed conducting oxide cathodes. This
implies that when doped LaMn03 is used for a cathode, oxygen will be
transported through the gaseous phase or on the surface of the LaMn03 to the
major electrochemical reaction sites at the electrolyte/cathode/gas three-phase
boundary as will be described in the next section. In the high overpotential
region, however, some authors have pointed out that oxide ion diffusion can
take place inside doped LaMn03 because the oxide ion vacancies can be formed at
the Iower oxygen potentials. This oxide ion flow could contribute to the
electrochemical reaction [3 94 11. Such oxygen diffusion inside the lanthanum
manganite was confirmed with '*O labelling and SIMS analysis [42]. LaGo03-
based oxides show higher oxide ion diffusivity and larger surface exchange
coefficients than the LaMnO3-based ones as listed in TabIe 5.2.
Although a detailed description of cathode reactions and polarisations is given
in Chapter 9, a brief discussion of the cathode reaction mechanisms is included
here to elucidate several aspects of materials issues in cathode development. As
discussed in the previous section, the cathode reduces oxygen molecules to