Page 155 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 155
132 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
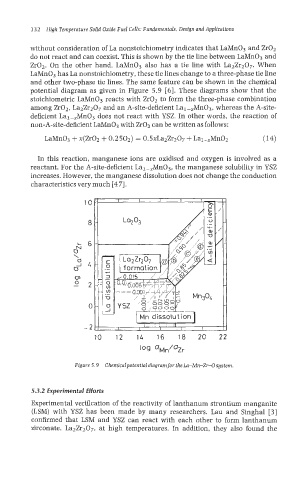
without consideration of La nonstoichiometry indicates that LaMn03 and Zr02
do not react and can coexist. This is shown by the tie line between LaMn03 and
Zr02. On the other hand, LaMn03 also has a tie line with LazZr2O7. When
LaMn03 has La nonstoichiometry, these tie lines change to a three-phase tie line
and other two-phase tie lines. The same feature can be shown in the chemical
potential diagram as given in Figure 5.9 [6]. These diagrams show that the
stoichiometric LaMn03 reacts with Zr02 to form the three-phase combination
among Zr02, La2Zr207 and an A-site-deficient Lal-,Mn03, whereas the A-site-
deficient Lal-,MnO3 does not react with YSZ. In other words, the reaction of
non-A-site-deficient LaMn03 with Zr02 can be written as follows:
LaMn03 + x(ZrO2 + 0.2502) = 0.5xLa2Zr207 + Lal-,MnO2 (14)
In this reaction, manganese ions are oxidised and oxygen is involved as a
reactant. For the A-site-deficient La1-,Mn03, the manganese solubility in YSZ
increases. However, the manganese dissolution does not change the conduction
characteristics very much [47].
10 I I I I I
8
~6
ON
\
d
-I4
b
cn
-02
0
Mn dissolution
-2 I,
Figure 5.9 Chemicalpotentialdiagram for the Ln-Mn-Zr-Osystem.
5.3.2 Experimental Efforts
Experimental verification of the reactivity of lanthanum strontium manganite
(LSM) with YSZ has been made by many researchers. Lau and Singhal [3]
confirmed that LSM and YSZ can react with each other to form lanthanum
zirconate, La2Zr207, at high temperatures. In addition, they also found the