Page 154 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 154
Cathodes 131
0.2V02.5 + (ZrO~)o~8(Y0~.~)o,2 0.8ZrO~(monoclinic) + 0.2YV04 (12)
=
The driving force for this reaction is the large stabilisation energy of YV04.
This can be categorised as the salt formation between an acidic oxide (V205) and
a basic oxide (Y203). Since yttria, which is the stabiliser of the cubic phase. is
extracted, this reaction leads to serious destabilisation of YSZ.
5.3.7.3 lnterdiffusion between Perovskite and Fluorite Oxides
Both perovslrite and fluorite oxides can form solid solutions with the common
oxide components allowing interdiffusion to take place. For example, manganese
ions can diffuse from the perovskite to the fluorite oxide:
Mn"+(in perovskite) + n/202- = Mnm' (in fluorite)
(13)
+ m/202- + (n - m)/202(g)
When alkali earth substituted lanthanum transition metal oxides are used as
cathodes, their compatibility with YSZ can be predicted from thermodynamic
considerations [6,45,46]. Such considerations show that LaCo03 (> 1173 K)
and LaNi03 are unstable against reaction (9). Similarly, alkali earth transition
metal oxides (ACo03, ACr03, AFe03, A = Ca, Sr, Ba) are unstable against the
reductive reactions. Also, although the thermodynamic data show that LaMnOs
is stable against the reactions (9), (lo), and (ll), experimental results do show
some reactions. This can be accounted for in terms of the lanthanum
nonstoichiometry in LaMn03. The reactivity with YSZ can be represented by a
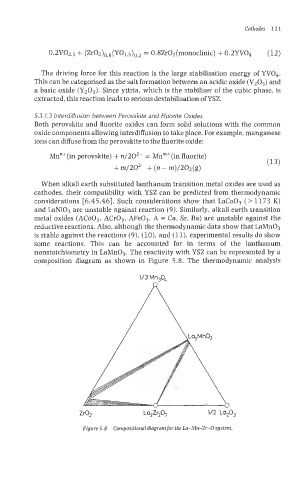
composition diagram as shown in Figure 5.8. The thermodynamic analysis
zfiz La,Zr,O, IM La,O,
Figure 5.8 Cornpositionaldiagramfor the La-Mn-Zr-0 system.