Page 151 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 151
128 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
Table 5.2 Oxygen diffusion coefficients (D? of LaMn03-based perovskites [32,35]
Composition Temperature P(02) Isotope oxygen Oxygen surface Ref.
(“C) (atm) diffusion coefficient, exchange coefficient,
D’ (cm2 s-l) k* (cm s-l)
900 1 2.44 x 10-13 32
1000 1 4.78 x 32
900 1 1.27 x 32
1000 1 1.33 x - 32
1000 1 6.60 x 10-13 5.62 x lovs 35
900 1 1.60 x 10-13 1.78 x 35
800 1 4.00 x 5.62 x 10-9 35
700 1 3.10 x 1O-l6 1.01 x 10-9 35
1000 1 9.01 x 10-8 5.64 x 35
900 1 1.03 x 2.02 x 10-6 35
8 00 1 9.87 x 6-31 x 10-7 35
700 1 1.04 x 10-lo 1.58 x lo-’ 35
oxide ions around the cathode/electrolyte interface. The elemental steps for
cathode reaction are: (i) oxygen molecule adsorption and dissociation into
oxygen atoms at the cathode surface, (ii) surface diffusion of adsorbed oxygen,
(iii) incorporation and subsequent bulk diffusion of oxygen inside the oxide
lattice, (iv) incorporation of adsorbed oxygen in the 02/cathode/electrolyte
three-phase boundary, and (v) transport of oxide ions in the solid electrolyte. The
charge transfer reaction can take place in steps (i), (iii), or (iv). Any of these
elemental steps can limit the rate of the cathodic reaction.
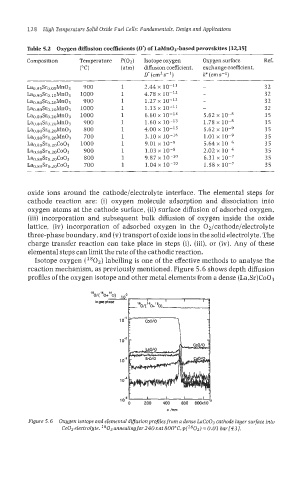
Isotope oxygen (l802) labelling is one of the effective methods to analyse the
reaction mechanism, as previously mentioned. Figure 5.6 shows depth diffusion
profiles of the oxygen isotope and other metal elements from a dense (La,Sr)Co03
F-
in gaS phase
160+’80)
1 0-1
1 o1
1 o-~
1 o4
1 o-~ 200 400 600 800x10
-
9
x /nm
Figure 5.6 Oxygen isotope and elemental difusion profiles from a dense LaCo03 cathode layer surface into
CeO, electrolyte. 2802 annealing for240 sat 800”C, p(l802) = 0.01 bar[43].