Page 158 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 158
Cathodes 135
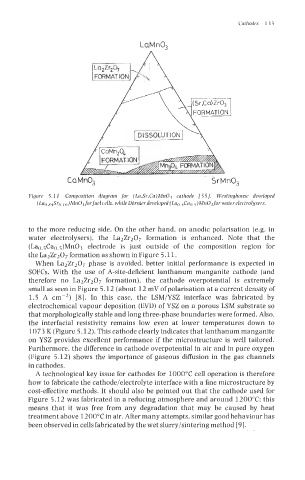
LaMn03
CaMn% SrMnO,
Figure 5.11 Composition diagram for (La,Sr,Ca)Mn03 cathode 1551. Westinghouse developed
(Lao x4Sro 16)Mn03 forfuel cells, while Dornier developed (Lao &ao 5)Mn03 for water electrolysers.
to the more reducing side. On the other hand, on anodic polarisation (e.g. in
water electrolysers), the La2Zr207 formation is enhanced. Note that the
(Lao.5Cao,5)Mn03 electrode is just outside of the composition region for
the La2Zr207 formation as shown in Figure 5.11.
When La2Zr207 phase is avoided, better initial performance is expected in
SOFCs. With the use of A-site-deficient lanthanum manganite cathode (and
therefore no La2Zr207 formation), the cathode overpotential is extremely
small as seen in Figure 5.12 (about 12 mV of polarisation at a current density of
1.5 A cm-2) [8]. In this case, the LSM/YSZ interface was fabricated by
electrochemical vapour deposition (EVD) of YSZ on a porous LSM substrate so
that morphologically stable and long three-phase boundaries were formed. Also,
the interfacial resistivity remains low even at lower temperatures down to
1073 K (Figure 5.12). This cathode clearly indicates that lanthanum manganite
on YSZ provides excellent performance if the microstructure is well tailored.
Furthermore, the difference in cathode overpotential in air and in pure oxygen
(Figure 5.12) shows the importance of gaseous diffusion in the gas channels
in cathodes.
A technological key issue for cathodes for 1000°C cell operation is therefore
how to fabricate the cathode/electrolyte interface with a fine microstructure by
cost-effective methods. It should also be pointed out that the cathode used for
Figure 5.12 was fabricated in a reducing atmosphere and around 1200°C; this
means that it was free from any degradation that may be caused by heat
treatment above 1200°C in air. After many attempts, similar good behaviour has
been observed in cells fabricated by the wet slurry/sintering method [9].