Page 157 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 157
134 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
0.
1 AIR ELECTRODE
-’.’ SINGLE CELL
-0.4 -
-. Cell size ; 6 minQ 0.3 cm’ 1
2
b
-0.8
-0.8
____ ___..--- . _”__.----- I
I ______.
--A_-
. .-
0 200 400 600 800 low) 12M) 1400
j I mA cm”
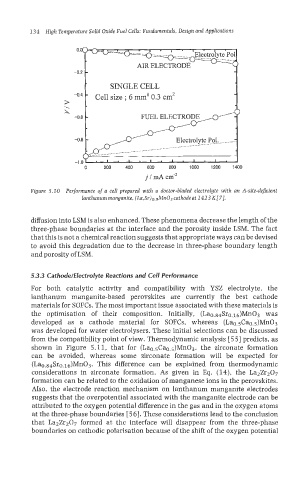
Figure 5-10 Performance of a cell prepared with a doctor-bladed electrolyte with an A-site-deficient
lanthanum manganite, (La,Sr)o.9MnO~ cathodeat 1423 K[7].
diffusion into LSM is also enhanced. These phenomena decrease the length of the
three-phase boundaries at the interface and the porosity inside LSM. The fact
that this is not a chemical reaction suggests that appropriate ways can be devised
to avoid this degradation due to the decrease in three-phase boundary length
and porosity of LSM.
5.3.3 Cathode/€lectrolyte Reactions and Cell Performance
For both catalytic activity and compatibility with YSZ electrolyte, the
lanthanum manganite-based perovsltites are currently the best cathode
materials for SOFCs. The most important issue associated with these materials is
the optimisation of their composition. Initially, (Lao.8&o.lb)Mn03 was
developed as a cathode material for SOFCs, whereas (Lao.5Cao.5)Mn03
was developed for water electrolysers. These initial selections can be discussed
from the compatibility point of view. Thermodynamic analysis [55] predicts, as
shown in Figure 5.11, that for (Lao.5Cao.5)Mn03, the zirconate formation
can be avoided, whereas some zirconate formation will be expected for
(Lao.8&0.16)Mn03. This difference can be explained from thermodynamic
considerations in zirconate formation. As given in Eq. (14), the La2Zr207
formation can be related to the oxidation of manganese ions in the perovskites.
Also, the electrode reaction mechanism on lanthanum manganite electrodes
suggests that the overpotential associated with the manganite electrode can be
attributed to the oxygen potential difference in the gas and in the oxygen atoms
at the three-phase boundaries [56]. These considerations lead to the conclusion
that La2Zr207 formed at the interface will disappear from the three-phase
boundaries on cathodic polarisation because of the shift of the oxygen potential