Page 45 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 45
2 6 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
were oxide ion conductors and the platinum contacts behaved as air electrodes.
It follows that Nernst lamps were the first commercially produced solid
electrolyte gas cells.
2.2 From Solid Electrolyte Gas Cells to Solid Oxide Fuel Cells
Electrochemistry was given an important impetus when its connection with
thermodynamics was explained by Helmholtz in 1882 [23]. Then, in 1894
Ostwald demonstrated that energy from coal could be produced much more
efficiently with a galvanic cell than with a steam engine [24].
The agreement between the voltages measured with galvanic solid electrolyte
gas cells and calculated thermodynamically was verified by Haber and
co-workers in 1905. From 3 30 to 5 70°C they used glass and from 800 to 1100°C
porcelain as the electrolyte, and partly platinum, partly gold as the material for
the electrodes in cells, first with C, CO, C02 and O2 [25], then in oxyhydrogen
cells, and in hydrogen and oxygen concentration cells [26,27]. Typical
phenomena such as the dependence of the voltage on the gas flux, deviations
from zero (‘asymmetry voltages’), and sluggishness in the establishment of
constant voltages at low temperatures were observed. Parallel to the publication
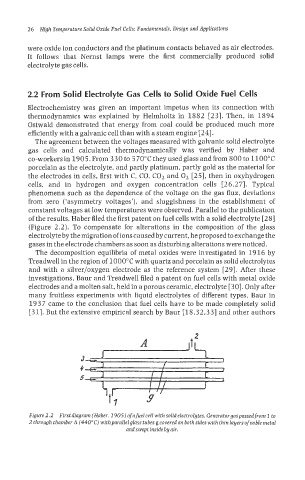
of the results, Haber filed the first patent on fuel cells with a solid electrolyte [28]
(Figure 2.2). To compensate for alterations in the composition of the glass
electrolyte by the migration ofions caused by current, he proposed to exchange the
gases in the electrode chambers as soon as disturbing alterations were noticed.
The decomposition equilibria of metal oxides were investigated in 1916 by
Treadwell in the region of 1000°C with quartz and porcelain as solid electrolytes
and with a silver/oxygen electrode as the reference system [29]. After these
investigations, Baur and Treadwell filed a patent on fuel cells with metal oxide
electrodes and a molten salt, held in a porous ceramic, electrolyte [30]. Only after
many fruitless experiments with liquid electrolytes of different types, Baur in
1937 came to the conclusion that fuel cells have to be made completely solid
[31]. But the extensive empirical search by Baur [18,32,33] and other authors
: 2
11 Y
Figure2.2 First diagram (Haber, 1905) of a fuel cell with solid electrolytes. Generatorgaspassedfrom 1 to
2 through chamber A (440°C) withparalleiglass tubes g covered on both sides with thin layers ofnoble metal
andsweptinsidebyair.