Page 47 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 47
28 High Temperature SoIid Oxide Fuel Cells: Fundamentals, Design and Applications
the cells by changing the gas supply. Concerning the electrolytes, for which
Schottky required a conductivity near 0.3 S/cm, halides, sulphates, carbonates
and phosphates were considered but no oxides. An electrochemical exploitation
of the combustion of coal seemed to be less feasible than that of the formation of
hydrogen chloride.
Zirconia ceramics were first used in fuel cells in 193 7 by Baur and Preis [18].
They wrote on the Degussa tube crucibles used (16 mm x 12 mm x 190 mm):
‘Unsurpassed is the Nernst mass. But even this mixture is not satisfactory
because the current enhances resistance considerably by electrolytic shift
(migration away of the cations).’ The problems were possibly caused by the
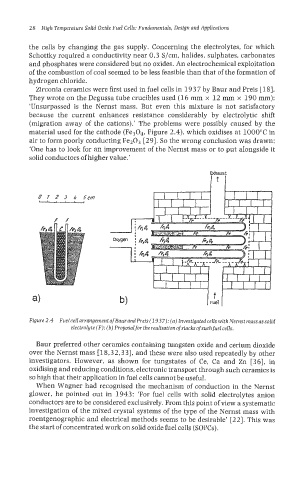
material used for the cathode (Fe304, Figure 2.4), which oxidises at 1000°C in
air to form poorly conducting Fez03 [29]. So the wrong conclusion was drawn:
‘One has to look for an improvement of the Nernst mass or to put alongside it
solid conductors of higher value.’
Figure 2.4 Fuel cell arrangement of Baurand Preis (I 937): (a) Investigated cells with Nernst mass as solid
electrolyte (F); (b) Proposalfor the realisation of stacks of such fuelcells.
Baur preferred other ceramics containing tungsten oxide and cerium dioxide
over the Nernst mass [18,32,33], and these were also used repeatedly by other
investigators. However, as shown for tungstates of Ce, Ca and Zn [36], in
oxidising and reducing conditions, electronic transport through such ceramics is
so high that their application in fuel cells cannot be useful.
When Wagner had recognised the mechanism of conduction in the Nernst
glower, he pointed out in 1943: ‘For fuel cells with solid electrolytes anion
conductors are to be considered exclusively. From this point of view a systematic
investigation of the mixed crystal systems of the type of the Nernst mass with
roentgenographic and electrical methods seems to be desirable’ [22]. This was
the start of concentrated work on solid oxide fuel cells (SOPCs).