Page 51 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 51
32 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
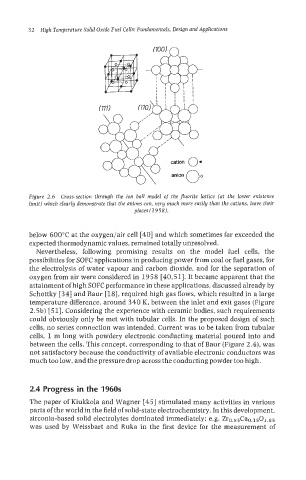
Figure 2.6 Cross-section through the ion ball model of the fluorite lattice (at the lower existence
limit) which clearly demonstrate that the anions can, very much more easily than the cations, leave their
places (1958).
below 600°C at the oxygen/air cell [40] and which sometimes far exceeded the
expected thermodynamic values, remained totally unresolved.
Nevertheless, following promising results on the model fuel cells, the
possibilities for SOFC applications in producing power from coal or fuel gases, for
the electrolysis of water vapour and carbon dioxide, and for the separation of
oxygen from air were considered in 1958 [40,51]. It became apparent that the
attainment of high SOFC performance in these applications, discussed already by
Schottky [34] and Baur [18], required high gas flows, which resulted in a large
temperature difference, around 340 K, between the inlet and exit gases (Figure
2.5b) [5 11. Considering the experience with ceramic bodies, such requirements
could obviously only be met with tubular cells. In the proposed design of such
cells, no series connection was intended. Current was to be taken from tubular
cells, 1 m long with powdery electronic conducting material poured into and
between the cells. This concept, corresponding to that of Baur (Figure 2.4), was
not satisfactory because the conductivity of available electronic conductors was
much too low, and the pressure drop across the conducting powder too high.
2.4 Progress in the 1960s
The paper of Kiuklrola and Wagner [45] stimulated many activities in various
parts of the world in the field of solid-state electrochemistry. In this development,
zirconia-based solid electrolytes dominated immediately: e.g. Zro.8 sCao.l
was used by Weissbart and Ruka in the first device for the measurement of