Page 46 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 46
History 27
up to the 1950s for suitable solid electrolytes, covering glasses, porcelains, clays
and a great variety of oxide mixtures, was unsuccessful.
The empirical phase of the development of solid electrolyte fuel ceIls was
overcome only after many general advances in research on solids. These included
development of X-ray structure analysis, new knowledge on the ion conduction of
solids from the measurements of transport numbers by Tubandt (first detection
of unipolar conduction by anions), the establishment of the theory of disorder
in sotids by Frenltel, Schottky, Wagner and Jost, and the development of isotope
methods for the investigation of diffusion processes in solids.
Starting from the observation of effects caused by small excesses of
components in salts and oxides, Schottky investigated problems of fuel cells with
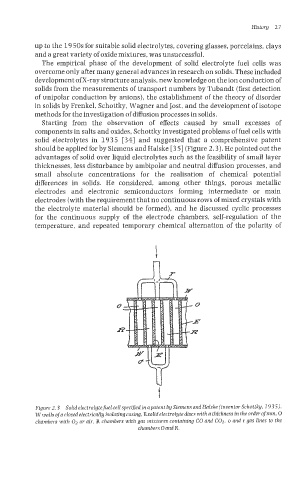
solid electrolytes in 1935 [34] and suggested that a comprehensive patent
should be applied for by Siemens and Halske [35] (Figure 2.3). He pointed out the
advantages of solid over liquid electrolytes such as the feasibility of small layer
thicknesses, less disturbance by ambipolar and neutral diffusion processes, and
small absolute concentrations for the realisation of chemical potential
differences in solids. He considered, among other things, porous metallic
electrodes and electronic semiconductors forming intermediate or main
electrodes (with the requirement that no continuous rows of mixed crystals with
the electrolyte material should be formed), and he discussed cyclic processes
for the continuous supply of the electrode chambers, self-regulation of the
temperature, and repeated temporary chemical alternation of the polarity of
t
Figure 2.3 Solid electrolgte fuel cell specijed in apatent by Siemens and Halske (inventor Schottkg, 2 935).
W walls ofa closed electrically isolating casing, E solid electrolyte discs with a thickness in the order ofmm, 0
chambers with O2 or air, R chambers with gas mixtures containing CO and CO2, o and r gas lines to the
chambers 0 and R.