Page 79 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 79
56 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
SH superheater
ST st.eam turbine
syst system
3.2 The Ideal Reversible SOFC
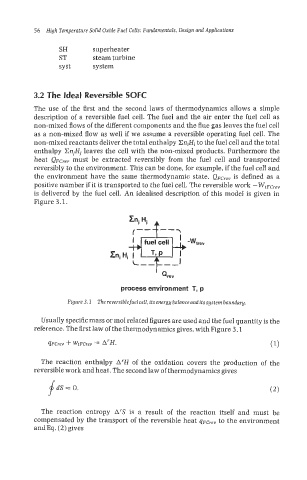
The use of the first and the second laws of thermodynamics allows a simple
description of a reversible fuel cell. The fuel and the air enter the fuel cell as
non-mixed flows of the different components and the flue gas leaves the fuel cell
as a non-mixed flow as well if we assume a reversible operating fuel cell. The
non-mixed reactants deliver the total enthalpy CniHi to the fuel cell and the total
enthalpy CnjHj leaves the cell with the non-mixed products. Furthermore the
heat QFcre,, must be extracted reversibly from the fuel cell and transported
reversibly to the environment. This can be done, for example, if the fuel cell and
the environment have the same thermodynamic state. QFCrev is defined as a
positive number if it is transported to the fuel cell. The reversible work - WtFCrev
is delivered by the fuel cell. An idealised description of this model is given in
Figure 3,l.
I
Q,
process environment T, p
Figure 3.2 The reversible fuelcell, its energy balanceanditssystem boundary.
Usually specific mass or mol related figures are used and the fuel quantity is the
reference. The first law of the thermodynamics gives, with Figure 3.1
qFCrev + WtFCrev = (1)
The reaction enthalpy A'H of the oxidation covers the production of the
reversible work and heat. The second law of thermodynamics gives
f
as = 0.
The reaction entropy ArS is a result of the reaction itself and must be
compensated by the transport of the reversible heat qFCrev to the environment
and Eq. (2) gives