Page 80 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 80
Thermodynamics 5 7
qFCrev -
0.
A'S - - (3)
-
TFC
Equations (1) and (3) give the reversible work wtFCrrv
The reversible work wtFCrrv the reaction is equal to the free or Gibbs enthalpy
of
ArG of the reaction
The reversible efficiency qFCrev of the fuel cell is the ratio of the Gibbs enthalpy
A'G and the reaction enthalpy A'H at the thermodynamic state of the fuel cell
and Eq. (5) gives
The process environment of a SOFC cannot exist near the ambient state and it
is thus an artificial model only. A general reversible SOFC system operation is
only possible within a system connecting reversibly the process environment
with the ambient state. This is the approach in Section 3.5.
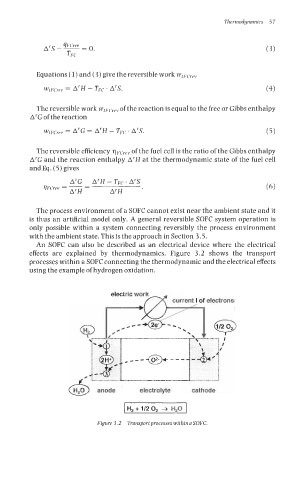
An SOFC can also be described as an electrical device where the electrical
effects are explained by thermodynamics. Figure 3.2 shows the transport
processes within a SOFC connecting the thermodynamic and the electrical effects
using the example of hydrogen oxidation.
H2 + 1/2 02 + H2O
Figure 3.2 Transportprocesses within a SOFC