Page 84 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 84
Thermodynamics 6 1
provides a first idea of the behaviour of real cells under changing operation
conditions. It can be assumed that the reaction enthalpy and the reaction
entropy depend only slightly on the temperature as a first assumption. Thus the
free enthalpy of the reaction at a higher temperature can be approximated with
the values of the reaction enthalpy and the reaction entropy at standard
conditions and it depends on the temperature linearly and yields the reversible
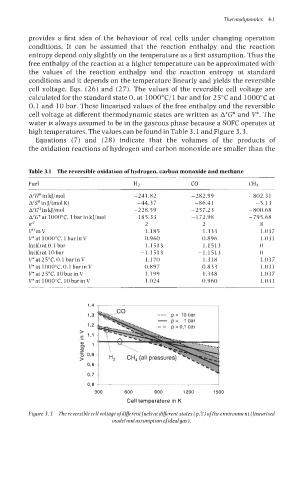
cell voltage, Eqs. (26) and (27). The values of the reversible cell voltage are
calculated for the standard state 0, at lOOO"C/l bar and for 25°C and 1000°C at
0.1 and 10 bar. These linearised values of the free enthalpy and the reversible
cell voltage at different thermodynamic states are written as A'G* and V*. The
water is always assumed to be in the gaseous phase because a SOFC operates at
high temperatures. The values can be found in Table 3.1 and Figure 3.3.
Equations (7) and (28) indicate that the volumes of the products of
the oxidation reactions of hydrogen and carbon monoxide are smaller than the
Table 3.1 The reversible oxidation of hydrogen, carbon monoxide and methane
Fuel H2 co CH4
A'P in kJ/mol -241.82 - 2 8 2.9 9 -802.31
A'S" in J/(mol K) -44.37 -86.41 -5.13
ArGOin kJ/mol -228.59 -257.23 -800.68
A'G* at lOOO"C, 1 bar in kJ/mol -185.33 -172.98 -795.68
ne' 2 2 8
V) in V 1.185 1.333 1.037
V* at 1000°C. 1 bar in V 0.960 0.896 1.031
In(K) at 0.1 bar 1.1513 1.1513 0
In(K) at 10 bar -1.1513 -1.1513 0
Vat25"C.O.l barinV 1.170 1.318 1.037
V at 1000°C. 0.1 bar in V 0.897 0.833 1.03 1
Vat25"C. 10barinV 1.199 1.348 1.037
V at 1000°C. 10 bar in V 1.024 0.960 1.031
1.4 ,
1 3 ...... p = 10 bar
- p= 1 bar
1 2
>
c 1,1
.-
% I
+- m
T5 03
> H, CH, (all pressures)
0.8
0,7
0,6 7
300 600 900 1200 1500
Cell temperature in K
Figure 3.3 The reversiblecell voltageofdiferentfuels at diferent states (p.T) ofthe environment (linearised
model andassumption ofidealgas).