Page 95 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 95
72 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
------- -- heat
fuel cell - fluid
work
I waste heat
I
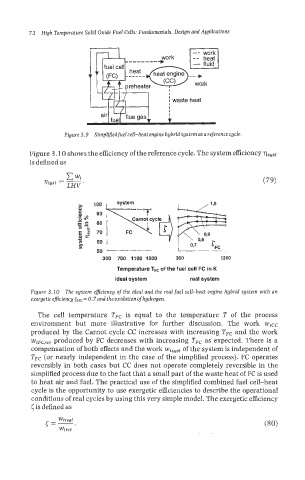
Figure 3.9 Simpl~edfuelcell-heat engine hybrid systemasa reference cycle.
Figure 3.10 shows the efficiency of the reference cycle. The system efficiency qsyst
is defined as
c
wt
rsyst = - (79)
LHV .
vd
cycle
Carnot
300 700 1100 1500 300 1300
Temperature TFc of the fuel cell FC in K
ideal system real system
Figure 3.20 The system efficiency of the ideal and the realfuel cell-heat engine hybrid system with an
exergetic eficiency cHE = 0.7 and the oxidation ofhydrogen.
The cell temperature TFc is equal to the temperature T of the process
environment but more illustrative for further discussion. The work wtCC
produced by the Carnot cycle CC increases with increasing TFc and the work
wtFCrev produced by FC decreases with increasing TFc as expected. There is a
compensation of both effects and the work wtsyst of the system is independent of
TFc (or nearly independent in the case of the simplified process). FC operates
reversibly in both cases but CC does not operate completely reversible in the
simplified process due to the fact that a small part of the waste heat of FC is used
to heat air and fuel. The practical use of the simplified combined fuel cell-heat
cycle is the opportunity to use exergetic efficiencies to describe the operational
conditions of real cycles by using this very simple model. The exergetic efficiency
cis defined as