Page 168 - How To Solve Word Problems In Calculus
P. 168
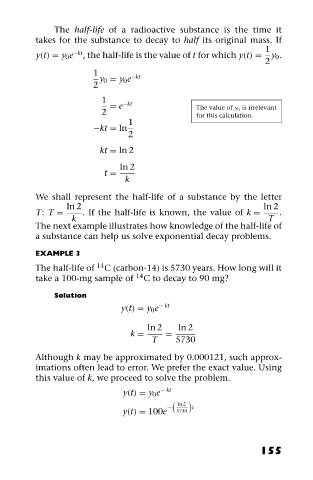
The half-life of a radioactive substance is the time it
takes for the substance to decay to half its original mass. If
1
y(t) = y 0 e −kt , the half-life is the value of t for which y(t) = y 0 .
2
1
y 0 = y 0 e −kt
2
1
= e −kt The value of y 0 is irrelevant
2 for this calculation.
1
−kt = ln
2
kt = ln 2
ln 2
t =
k
We shall represent the half-life of a substance by the letter
ln 2 ln 2
T: T = . If the half-life is known, the value of k = .
k T
The next example illustrates how knowledge of the half-life of
a substance can help us solve exponential decay problems.
EXAMPLE 3
The half-life of 14 C (carbon-14) is 5730 years. How long will it
take a 100-mg sample of 14 C to decay to 90 mg?
Solution
y(t) = y 0 e − kt
ln 2 ln 2
k = =
T 5730
Although k may be approximated by 0.000121, such approx-
imations often lead to error. We prefer the exact value. Using
this value of k, we proceed to solve the problem.
y(t) = y 0 e − kt
ln 2 t
y(t) = 100e − 5730
155