Page 136 - Hydrocarbon Exploration and Production Second Edition
P. 136
Reservoir Description 123
400 X I.C. gas condensate &
oil reservoirs
300
Pressure (10 5 Pa) 200 reservoir
X
I. C.
gas
100
separator
dry wet gas
gas gas condensate volatile oil black oil
0
-100 0 100 200 300 400
Temperature (°C)
= critical point I.C. = initial conditions
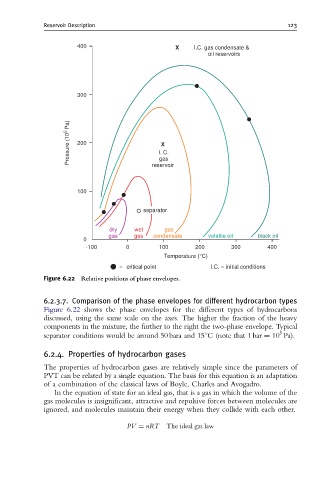
Figure 6.22 Relative positions of phase envelopes.
6.2.3.7. Comparison of the phase envelopes for different hydrocarbon types
Figure 6.22 shows the phase envelopes for the different types of hydrocarbons
discussed, using the same scale on the axes. The higher the fraction of the heavy
components in the mixture, the further to the right the two-phase envelope. Typical
5
separator conditions would be around 50 bara and 151C (note that 1 bar ¼ 10 Pa).
6.2.4. Properties of hydrocarbon gases
The properties of hydrocarbon gases are relatively simple since the parameters of
PVT can be related by a single equation. The basis for this equation is an adaptation
of a combination of the classical laws of Boyle, Charles and Avogadro.
In the equation of state for an ideal gas, that is a gas in which the volume of the
gas molecules is insignificant, attractive and repulsive forces between molecules are
ignored, and molecules maintain their energy when they collide with each other.
PV ¼ nRT The ideal gas law