Page 150 - Hydrocarbon Exploration and Production Second Edition
P. 150
Reservoir Description 137
Similarly, when drilling into an underpressured formation, the mud weight must
be reduced to avoid excessive losses into the formation. If the rate of loss is greater
than the rate at which mud can be made up, then the level of fluid in the wellbore will
drop and there is a risk of influx from the normally pressured overlying formations.
Again, it may be necessary to set a casing before drilling into underpressures.
6.2.9. Capillary pressure and saturation–height relationships
In a reservoir at initial conditions, an equilibrium exists between buoyancy forces
and capillary forces. These forces determine the initial distribution of fluids, and
hence the volumes of fluid in place. An understanding of the relationship between
these forces is useful in calculating volumetrics, and in explaining the difference
between FWL and OWC introduced in Section 6.2.8.1.
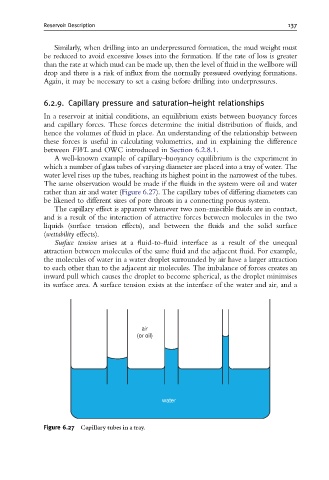
A well-known example of capillary–buoyancy equilibrium is the experiment in
which a number of glass tubes of varying diameter are placed into a tray of water. The
water level rises up the tubes, reaching its highest point in the narrowest of the tubes.
The same observation would be made if the fluids in the system were oil and water
rather than air and water (Figure 6.27). The capillary tubes of differing diameters can
be likened to different sizes of pore throats in a connecting porous system.
The capillary effect is apparent whenever two non-miscible fluids are in contact,
and is a result of the interaction of attractive forces between molecules in the two
liquids (surface tension effects), and between the fluids and the solid surface
(wettability effects).
Surface tension arises at a fluid-to-fluid interface as a result of the unequal
attraction between molecules of the same fluid and the adjacent fluid. For example,
the molecules of water in a water droplet surrounded by air have a larger attraction
to each other than to the adjacent air molecules. The imbalance of forces creates an
inward pull which causes the droplet to become spherical, as the droplet minimises
its surface area. A surface tension exists at the interface of the water and air, and a
air
(or oil)
water
Figure 6.27 Capillary tubes in a tray.