Page 134 - Hydrogeology Principles and Practice
P. 134
HYDC03 12/5/05 5:37 PM Page 117
Chemical hydrogeology 117
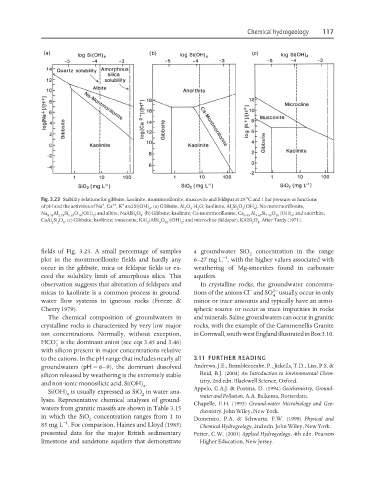
Fig. 3.23 Stability relations for gibbsite, kaolinite, montmorillonite, muscovite and feldspar at 25°C and 1 bar pressure as functions
+ 2+ +
of pH and the activities of Na , Ca , K and Si(OH) . (a) Gibbsite, Al O ⋅H O; kaolinite, Al Si O (OH ); Na-montmorillonite,
4 2 3 2 2 2 5 4
Na Al Si O (OH) ; and albite, NaAlSi O . (b) Gibbsite; kaolinite; Ca-montmorillonite, Ca Al Si O (OH) ; and anorthite,
0.33 2.33 3.67 10 2 3 8 0.33 4.67 7.33 20 4
CaAl Si O . (c) Gibbsite; kaolinite; muscovite, KAl (AlSi O )(OH) ; and microcline (feldspar), KAlSi O . After Tardy (1971).
2 2 8 2 3 10 2 3 8
fields of Fig. 3.23. A small percentage of samples a groundwater SiO concentration in the range
2
−1
plot in the montmorillonite fields and hardly any 6–27 mg L , with the higher values associated with
occur in the gibbsite, mica or feldspar fields or ex- weathering of Mg-smectites found in carbonate
ceed the solubility limit of amorphous silica. This aquifers.
observation suggests that alteration of feldspars and In crystalline rocks, the groundwater concentra-
− 2−
micas to kaolinite is a common process in ground- tions of the anions Cl and SO usually occur in only
4
water flow systems in igneous rocks (Freeze & minor or trace amounts and typically have an atmo-
Cherry 1979). spheric source or occur as trace impurities in rocks
The chemical composition of groundwaters in and minerals. Saline groundwaters can occur in granitic
crystalline rocks is characterized by very low major rocks, with the example of the Carnmenellis Granite
ion concentrations. Normally, without exception, in Cornwall, south-west England illustrated in Box 3.10.
−
HCO is the dominant anion (see eqs 3.45 and 3.46)
3
with silicon present in major concentrations relative
to the cations. In the pH range that includes nearly all 3.11 FURTHER READING
groundwaters (pH = 6–9), the dominant dissolved Andrews, J.E., Brimblecombe, P., Jickells, T.D., Liss, P.S. &
silicon released by weathering is the extremely stable Reid, B.J. (2004) An Introduction to Environmental Chem-
and non-ionic monosilicic acid, Si(OH) . istry, 2nd edn. Blackwell Science, Oxford.
4 Appelo, C.A.J. & Postma, D. (1994) Geochemistry, Ground-
Si(OH) is usually expressed as SiO in water ana-
4 2 water and Pollution. A.A. Balkema, Rotterdam.
lyses. Representative chemical analyses of ground-
Chapelle, F.H. (1993) Ground-water Microbiology and Geo-
waters from granitic massifs are shown in Table 3.15
chemistry. John Wiley, New York.
in which the SiO concentration ranges from 1 to
2 Domenico, P.A. & Schwartz, F.W. (1998) Physical and
−1
85 mg L . For comparison, Haines and Lloyd (1985) Chemical Hydrogeology, 2nd edn. John Wiley, New York.
presented data for the major British sedimentary Fetter, C.W. (2001) Applied Hydrogeology, 4th edn. Pearson
limestone and sandstone aquifers that demonstrate Higher Education, New Jersey.