Page 130 - Hydrogeology Principles and Practice
P. 130
HYDC03 12/5/05 5:37 PM Page 113
Chemical hydrogeology 113
BO X
Microbially mediated denitrification
3.9
The sequences of redox reactions shown in Tables 3.11 and 3.12 elements required in large amounts (C, H, O, N, P, S), minor amounts
are thermodynamically favoured, but their reaction rates are slow of minerals (K, Na, Mg, Ca, Fe) and trace amounts of certain metals
in the absence of catalysts. In natural waters, the most important (Mn, Zn, Cu, Co and Mo). On the basis of average cellular composi-
electron-transfer mechanism is catalysis associated with micro- tion, the favourable ratio of C : N : P : S is about 100 : 20 : 4 : 1
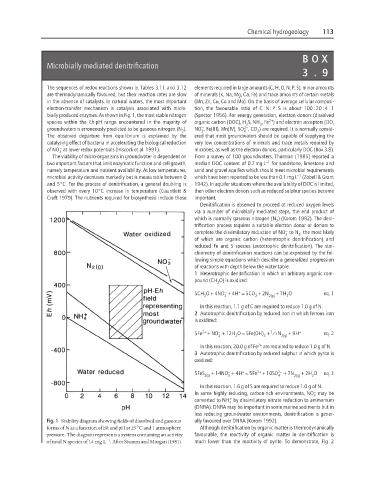
bially produced enzymes. As shown in Fig. 1, the most stable nitrogen (Spector 1956). For energy generation, electron donors (dissolved
2+
species within the Eh-pH range encountered in the majority of organic carbon (DOC), H S, NH , Fe ) and electron acceptors (DO,
2
3
−
2−
groundwaters is erroneously predicted to be gaseous nitrogen (N ). NO , Fe(III), Mn(IV), SO , CO ) are required. It is normally consid-
2
3
2
4
The observed departure from equilibrium is explained by the ered that most groundwaters should be capable of supplying the
catalysing effect of bacteria in accelerating the biological reduction very low concentrations of minerals and trace metals required by
−
of NO at lower redox potentials (Hiscock et al. 1991). microbes, as well as the electron donors, particularly DOC (Box 3.8).
3
The viability of micro-organisms in groundwater is dependent on From a survey of 100 groundwaters, Thurman (1985) reported a
two important factors that limit enzymatic function and cell growth, median DOC content of 0.7 mg L −1 for sandstone, limestone and
namely temperature and nutrient availability. At low temperatures, sand and gravel aquifers which should meet microbial requirements
−1
microbial activity decreases markedly but is measurable between 0 which have been reported to be less than 0.1 mg L (Zobell & Grant
and 5°C. For the process of denitrification, a general doubling is 1942). In aquifer situations where the availability of DOC is limited,
observed with every 10°C increase in temperature (Gauntlett & then other electron donors such as reduced sulphur species become
Craft 1979). The nutrients required for biosynthesis include those important.
Denitrification is observed to proceed at reduced oxygen levels
via a number of microbially mediated steps, the end product of
which is normally gaseous nitrogen (N ) (Korom 1992). The deni-
2
trification process requires a suitable electron donor or donors to
−
complete the dissimilatory reduction of NO to N , the most likely
3
2
of which are organic carbon (heterotrophic denitrification) and
reduced Fe and S species (autotrophic denitrification). The stoi-
chiometry of denitrification reactions can be expressed by the fol-
lowing simple equations which describe a generalized progression
of reactions with depth below the water table:
1 Heterotrophic denitrification in which an arbitrary organic com-
pound (CH O) is oxidized:
2
+
−
5CH O + 4NO + 4H = 5CO + 2N 2(g) + 7H O eq. 1
2
3
2
2
In this reaction, 1.1 g of C are required to reduce 1.0 g of N.
2 Autotrophic denitrification by reduced iron in which ferrous iron
is oxidized:
2+
−
1
5Fe + NO + 12H O = 5Fe(OH) + /2N 2(g) + 9H + eq. 2
3
2
3
2+
In this reaction, 20.0 g of Fe are required to reduce 1.0 g of N.
3 Autotrophic denitrification by reduced sulphur in which pyrite is
oxidized:
2+
+
−
5FeS 2(s) + 14NO + 4H = 5Fe + 10SO 2− + 7N 2(g) + 2H O eq. 3
3
2
4
In this reaction, 1.6 g of S are required to reduce 1.0 g of N.
−
In some highly reducing, carbon-rich environments, NO may be
3
+
converted to NH by dissimilatory nitrate reduction to ammonium
4
(DNRA). DNRA may be important in some marine sediments but in
less reducing groundwater environments, denitrification is gener-
Fig. 1 Stability diagram showing fields of dissolved and gaseous ally favoured over DNRA (Korom 1992).
forms of N as a function of Eh and pH at 25°C and 1 atmosphere Although denitrification by organic matter is thermodynamically
pressure. The diagram represents a system containing an activity favourable, the reactivity of organic matter in denitrification is
−1
of total N species of 14 mg L . After Stumm and Morgan (1981). much lower than the reactivity of pyrite. To demonstrate, Fig. 2