Page 131 - Hydrogeology Principles and Practice
P. 131
HYDC03 12/5/05 5:37 PM Page 114
114 Chapter Three
BO X
Continued
3.9
−
shows depth profiles for pH and the redox-sensitive species NO ,
3
2+
SO 4 2− and Fe for a multilevel observation well installed near to the
Vierlingsbeek wellfield in the south-east of the Netherlands. The
unconfined aquifer at this site is composed of unconsolidated
fluvial sands containing no calcite and low amounts of organic mat-
ter (0.1–2%) and pyrite (<0.01–0.2%), the amounts of which
decrease with depth (Fig. 3). An aquitard composed of fine-grained
cemented deposits exists at 30 m below ground level (m bgl) and
the water table varies between 2 and 4 m bgl. Very intensive cattle
farming in the vicinity of the wellfield with the application of liquid
−
manure has provided a source of high NO concentrations in the
3
shallow groundwater (van Beek 2000).
At Vierlingsbeek, at 10 m bgl, Fig. 2 shows that measured NO 3 −
concentrations are above 200 mg L −1 but that below 21 m bgl
−
−
NO is absent. The decrease in NO coincides with an increase in
3
3
SO 4 2− and Fe 2+ between 20 and 21 m bgl that can be explained by
autotrophic denitrification in which pyrite is oxidized (eq. 3). In this
reaction, and in the absence of carbonate to act as a buffer, protons
are consumed and a steady rise in pH is observed below 20 m bgl.
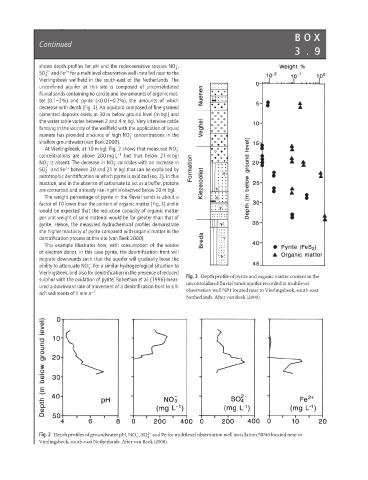
The weight percentage of pyrite in the fluvial sands is about a
factor of 10 lower than the content of organic matter (Fig. 3) and it
would be expected that the reduction capacity of organic matter
per unit weight of solid material would be far greater than that of
pyrite. Hence, the measured hydrochemical profiles demonstrate
the higher reactivity of pyrite compared with organic matter in the
denitrification process at this site (van Beek 2000).
This example illustrates how, with consumption of the source
of electron donor, in this case pyrite, the denitrification front will
migrate downwards such that the aquifer will gradually loose the
−
ability to attenuate NO . For a similar hydrogeological situation to
3
Vierlingsbeek, and also for denitrification in the presence of reduced
Fig. 3 Depth profile of pyrite and organic matter content in the
sulphur with the oxidation of pyrite, Robertson et al. (1996) meas-
unconsolidated fluvial sands aquifer recorded at multilevel
ured a downward rate of movement of a denitrification front in silt- observation well NP1 located near to Vierlingsbeek, south-east
−1
rich sediments of 1 mm a .
Netherlands. After van Beek (2000).
− 2−
Fig. 2 Depth profiles of groundwater pH, NO , SO and Fe for multilevel observation well installation NP40 located near to
3 4
Vierlingsbeek, south-east Netherlands. After van Beek (2000).