Page 235 - Hydrogeology Principles and Practice
P. 235
HYDC06 12/5/05 5:33 PM Page 218
218 Chapter Six
phase (Fig. 6.10). During simultaneous flow of two
immiscible fluids, and as shown in Fig. 6.10, part of
the available pore space will be filled with water and
the remainder with oil such that the cross-sectional
area of the pore space available for each fluid is less
than the total pore space. This situation leads to the
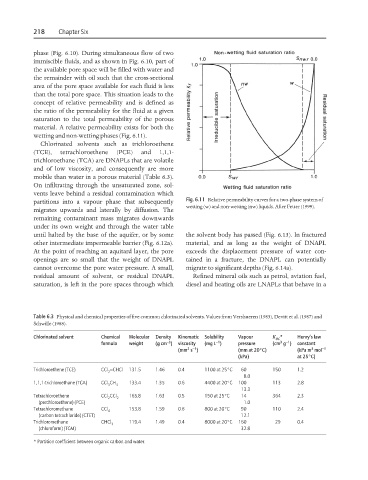
concept of relative permeability and is defined as
the ratio of the permeability for the fluid at a given
saturation to the total permeability of the porous
material. A relative permeability exists for both the
wetting and non-wetting phases (Fig. 6.11).
Chlorinated solvents such as trichloroethene
(TCE), tetrachloroethene (PCE) and 1,1,1-
trichloroethane (TCA) are DNAPLs that are volatile
and of low viscosity, and consequently are more
mobile than water in a porous material (Table 6.3).
On infiltrating through the unsaturated zone, sol-
vents leave behind a residual contamination which
partitions into a vapour phase that subsequently Fig. 6.11 Relative permeability curves for a two-phase system of
wetting (w) and non-wetting (nw) liquids. After Fetter (1999).
migrates upwards and laterally by diffusion. The
remaining contaminant mass migrates downwards
under its own weight and through the water table
until halted by the base of the aquifer, or by some the solvent body has passed (Fig. 6.13). In fractured
other intermediate impermeable barrier (Fig. 6.12a). material, and as long as the weight of DNAPL
At the point of reaching an aquitard layer, the pore exceeds the displacement pressure of water con-
openings are so small that the weight of DNAPL tained in a fracture, the DNAPL can potentially
cannot overcome the pore water pressure. A small, migrate to significant depths (Fig. 6.14a).
residual amount of solvent, or residual DNAPL Refined mineral oils such as petrol, aviation fuel,
saturation, is left in the pore spaces through which diesel and heating oils are LNAPLs that behave in a
Table 6.3 Physical and chemical properties of five common chlorinated solvents. Values from Vershueren (1983), Devitt et al. (1987) and
Schwille (1988).
Chlorinated solvent Chemical Molecular Density Kinematic Solubility Vapour K * Henry’s law
OC
−3
−1
3
−1
formula weight (g cm ) viscosity (mg L ) pressure (cm g ) constant
3
2 −1
(mm s ) (mm at 20°C) (kPa m mol −1
(kPa) at 25°C)
Trichloroethene (TCE) CCl =CHCl 131.5 1.46 0.4 1100 at 25°C 60 150 1.2
2
8.0
1,1,1-trichloroethane (TCA) CCl CH 133.4 1.35 0.6 4400 at 20°C 100 113 2.8
3 3
13.3
Tetrachloroethene CCl CCl 165.8 1.63 0.5 150 at 25°C 14 364 2.3
2 2
(perchloroethene) (PCE) 1.0
Tetrachloromethane CCl 153.8 1.59 0.6 800 at 20°C 90 110 2.4
4
(carbon tetrachloride) (CTET) 12.1
Trichloromethane CHCl 119.4 1.49 0.4 8000 at 20°C 160 29 0.4
3
(chloroform) (TCM) 32.8
* Partition coefficient between organic carbon and water.