Page 182 - Illustrated Pocket Dictionary of Chromatography
P. 182
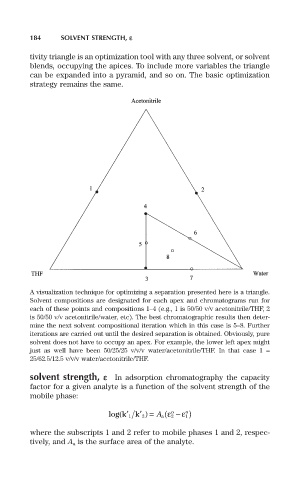
184 SOLVENT STRENGTH, e
tivity triangle is an optimization tool with any three solvent, or solvent
blends, occupying the apices. To include more variables the triangle
can be expanded into a pyramid, and so on. The basic optimization
strategy remains the same.
A visualization technique for optimizing a separation presented here is a triangle.
Solvent compositions are designated for each apex and chromatograms run for
each of these points and compositions 1–4 (e.g., 1 is 50/50 v/v acetonitrile/THF, 2
is 50/50 v/v acetonitrile/water, etc). The best chromatographic results then deter-
mine the next solvent compositional iteration which in this case is 5–8. Further
iterations are carried out until the desired separation is obtained. Obviously, pure
solvent does not have to occupy an apex. For example, the lower left apex might
just as well have been 50/25/25 v/v/v water/acetonitrile/THF. In that case 1 =
25/62.5/12.5 v/v/v water/acetonitrile/THF.
solvent strength, e In adsorption chromatography the capacity
factor for a given analyte is a function of the solvent strength of the
mobile phase:
o
log k 1 ¢ k 2 ¢ ) = A a (e 2 o - )
(
e 1
where the subscripts 1 and 2 refer to mobile phases 1 and 2, respec-
tively, and A a is the surface area of the analyte.