Page 187 - Illustrated Pocket Dictionary of Chromatography
P. 187
SUPERCRITICAL FLUID (SCF) 189
that are reacted. Hence, all other things equal, it is expected that there
would be more unreacted silanols on a non-endcapped C18 support
than a corresponding butyl support.
Stokes radius Also called the hydrodynamic radius, the Stokes
radius is the effective radius of a molecule in the solvent, including
any associated solvent molecules.
storage solvent Used to keep the packing material surface wetted
and protected from contamination. Manufacturers typically recom-
mend the type of storage solvent that should be used with their
columns. For example, an ion-exchange column storage column will
most likely have a defined salt type and concentration whereas a C18
bonded phase will use an organic solvent (such as acetonitrile, etc).
A very important aspect of the storage solvent is to prevent air from
entering the packing bed.
surface coverage The percent of active surface sites (e.g., silanol
groups) that are converted to the bonded phase. This is typically
2
derived from the theoretical number of silanol sites/m for the surface
2
(~8mmol silanol/m ).
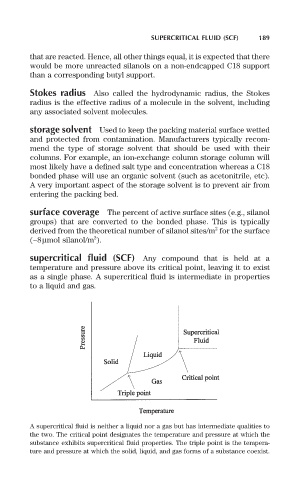
supercritical fluid (SCF) Any compound that is held at a
temperature and pressure above its critical point, leaving it to exist
as a single phase. A supercritical fluid is intermediate in properties
to a liquid and gas.
A supercritical fluid is neither a liquid nor a gas but has intermediate qualities to
the two. The critical point designates the temperature and pressure at which the
substance exhibits supercritical fluid properties. The triple point is the tempera-
ture and pressure at which the solid, liquid, and gas forms of a substance coexist.