Page 9 - Illustrated Pocket Dictionary of Chromatography
P. 9
2 ACETONE
viscosity (15°C): 1.31cP; polarity index (P¢): 6.2; Hildebrand solubility
parameter (d): 12.4; pK a = 4.7. Miscible with water. Acetic acid is a
weak acid that is frequently used as a mobile-phase modifier directly
or as a buffer (acetic acid/acetate) in LC, TLC, and CE techniques.
Pungent odor.
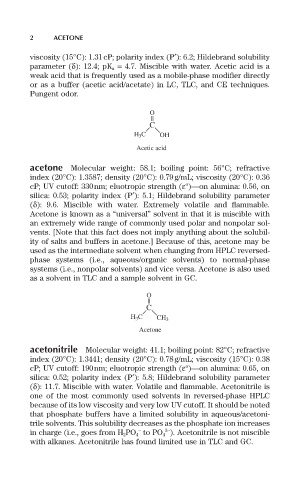
O
C
H 3 C OH
Acetic acid
acetone Molecular weight: 58.1; boiling point: 56°C; refractive
index (20°C): 1.3587; density (20°C): 0.79g/mL; viscosity (20°C): 0.36
o
cP; UV cutoff: 330nm; eluotropic strength (e )—on alumina: 0.56, on
silica: 0.53; polarity index (P¢): 5.1; Hildebrand solubility parameter
(d): 9.6. Miscible with water. Extremely volatile and flammable.
Acetone is known as a “universal” solvent in that it is miscible with
an extremely wide range of commonly used polar and nonpolar sol-
vents. [Note that this fact does not imply anything about the solubil-
ity of salts and buffers in acetone.] Because of this, acetone may be
used as the intermediate solvent when changing from HPLC reversed-
phase systems (i.e., aqueous/organic solvents) to normal-phase
systems (i.e., nonpolar solvents) and vice versa. Acetone is also used
as a solvent in TLC and a sample solvent in GC.
O
C
H 3 C CH 3
Acetone
acetonitrile Molecular weight: 41.1; boiling point: 82°C; refractive
index (20°C): 1.3441; density (20°C): 0.78g/mL; viscosity (15°C): 0.38
o
cP; UV cutoff: 190nm; eluotropic strength (e )—on alumina: 0.65, on
silica: 0.52; polarity index (P¢): 5.8; Hildebrand solubility parameter
(d): 11.7. Miscible with water. Volatile and flammable. Acetonitrile is
one of the most commonly used solvents in reversed-phase HPLC
because of its low viscosity and very low UV cutoff. It should be noted
that phosphate buffers have a limited solubility in aqueous/acetoni-
trile solvents. This solubility decreases as the phosphate ion increases
3-
-
in charge (i.e., goes from H 2 PO 4 to PO 4 ). Acetonitrile is not miscible
with alkanes. Acetonitrile has found limited use in TLC and GC.