Page 12 - Illustrated Pocket Dictionary of Chromatography
P. 12
ADSORPTION CHROMATOGRAPHY 5
ically slow, or irreversible. It is controlled by one or more interactions:
hydrogen bonding and van der Waals interactions. The condition of
rapid, reversible thermodynamic equilibrium is the desired one in
most separations.
adsorption chromatography Adsorption chromatography,
also called liquid-solid chromatography (LSC), uses unmodified solid
adsorbents such as silica, alumina, and carbon to generate a
separation. The retention mechanism for adsorption chromatography
is through the reversible equilibrium displacement of mobile-phase
components, M, with the analyte, A, on the surface of the adsorbent,
s, into the mobile phase, m:
A m + M s ´ A s + M m
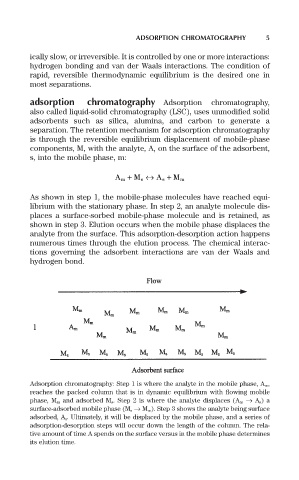
As shown in step 1, the mobile-phase molecules have reached equi-
librium with the stationary phase. In step 2, an analyte molecule dis-
places a surface-sorbed mobile-phase molecule and is retained, as
shown in step 3. Elution occurs when the mobile phase displaces the
analyte from the surface. This adsorption-desorption action happens
numerous times through the elution process. The chemical interac-
tions governing the adsorbent interactions are van der Waals and
hydrogen bond.
Adsorption chromatography: Step 1 is where the analyte in the mobile phase, A m ,
reaches the packed column that is in dynamic equilibrium with flowing mobile
phase, M m and adsorbed M s . Step 2 is where the analyte displaces (A m Æ A s ) a
surface-adsorbed mobile phase (M s Æ M m ). Step 3 shows the analyte being surface
adsorbed, A s . Ultimately, it will be displaced by the mobile phase, and a series of
adsorption-desorption steps will occur down the length of the column. The rela-
tive amount of time A spends on the surface versus in the mobile phase determines
its elution time.