Page 16 - Illustrated Pocket Dictionary of Chromatography
P. 16
AMPEROMETRIC DETECTOR 9
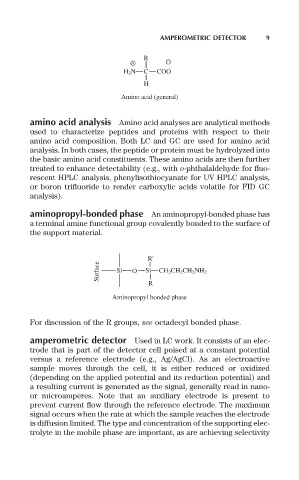
R
H 3 N C COO
H
Amino acid (general)
amino acid analysis Amino acid analyses are analytical methods
used to characterize peptides and proteins with respect to their
amino acid composition. Both LC and GC are used for amino acid
analysis. In both cases, the peptide or protein must be hydrolyzed into
the basic amino acid constituents. These amino acids are then further
treated to enhance detectability (e.g., with o-phthalaldehyde for fluo-
rescent HPLC analysis, phenylisothiocyanate for UV HPLC analysis,
or boron trifluoride to render carboxylic acids volatile for FID GC
analysis).
aminopropyl-bonded phase An aminopropyl-bonded phase has
a terminal amine functional group covalently bonded to the surface of
the support material.
R'
Surface Si O Si CH 2 CH 2 CH 2 NH 2
R
Aminopropyl bonded phase
For discussion of the R groups, see octadecyl bonded phase.
amperometric detector Used in LC work. It consists of an elec-
trode that is part of the detector cell poised at a constant potential
versus a reference electrode (e.g., Ag/AgCl). As an electroactive
sample moves through the cell, it is either reduced or oxidized
(depending on the applied potential and its reduction potential) and
a resulting current is generated as the signal, generally read in nano-
or microamperes. Note that an auxiliary electrode is present to
prevent current flow through the reference electrode. The maximum
signal occurs when the rate at which the sample reaches the electrode
is diffusion limited. The type and concentration of the supporting elec-
trolyte in the mobile phase are important, as are achieving selectivity