Page 15 - Illustrated Pocket Dictionary of Chromatography
P. 15
8 ALKENES
alkenes A class of compounds that are saturated hydrocarbons.
The general formula of a linear alkane is C nH 2n. Alkenes may also have
branched or cyclic structures. Alkenes do not have wide use as sol-
vents in chromatographic separations but find use as “preservatives”
for chlorinated alkane solvents (such as methylene chloride). For this
use, amylene (2-methyl-2-butene) and cyclohexene are examples.
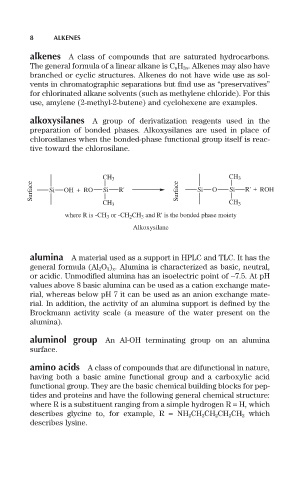
alkoxysilanes A group of derivatization reagents used in the
preparation of bonded phases. Alkoxysilanes are used in place of
chlorosilanes when the bonded-phase functional group itself is reac-
tive toward the chlorosilane.
CH 3 CH 3
Surface Si OH + RO Si R' Surface Si O Si R' + ROH
CH 3 CH 3
where R is -CH 3 or -CH 2 CH 3 and R' is the bonded phase moiety
Alkoxysilane
alumina A material used as a support in HPLC and TLC. It has the
general formula (Al 2O 3) x. Alumina is characterized as basic, neutral,
or acidic. Unmodified alumina has an isoelectric point of ~7.5. At pH
values above 8 basic alumina can be used as a cation exchange mate-
rial, whereas below pH 7 it can be used as an anion exchange mate-
rial. In addition, the activity of an alumina support is defined by the
Brockmann activity scale (a measure of the water present on the
alumina).
aluminol group An Al-OH terminating group on an alumina
surface.
amino acids A class of compounds that are difunctional in nature,
having both a basic amine functional group and a carboxylic acid
functional group. They are the basic chemical building blocks for pep-
tides and proteins and have the following general chemical structure:
where R is a substituent ranging from a simple hydrogen R = H, which
describes glycine to, for example, R = NH 2CH 2CH 2CH 2CH 2 which
describes lysine.