Page 317 - Industrial Ventilation Design Guidebook
P. 317
5,3 TOXIC1TY AND RISKS INDUCED BY OCCUPATIONAL EXPOSURE TO CHEMICAL COMPOUNDS 273
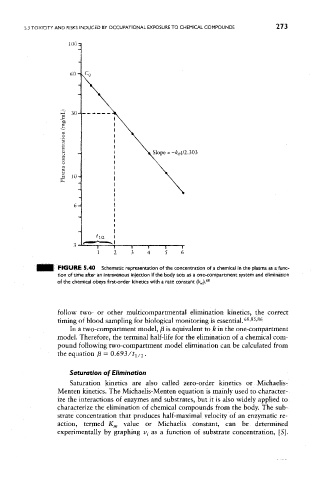
FIGURE 5.40 Schematic representation of the concentration of a chemical in the plasma as a func-
tion of time after an intravenous injection if the body acts as a one-compartment system and elimination
of the chemical obeys first-order kinetics with a rate constant (k e/). 68
follow two- or other multicompartmental elimination kinetics, the correct
68 85 86
timing of blood sampling for biological monitoring is essential. ' '
In a two-compartment model, /3 is equivalent to k in the one-compartment
model. Therefore, the terminal half-life for the elimination of a chemical com-
pound following two-compartment model elimination can be calculated from
the equation /3 = 0.693/£ 1/2 •
Saturation of Elimination
Saturation kinetics are also called zero-order kinetics or Michaelis-
Menten kinetics. The Michaelis-Menten equation is mainly used to character-
ize the interactions of enzymes and substrates, but it is also widely applied to
characterize the elimination of chemical compounds from the body. The sub-
strate concentration that produces half-maximal velocity of an enzymatic re-
action, termed K m value or Michaelis constant, can be determined
experimentally by graphing v i as a function of substrate concentration, [5].