Page 318 - Industrial Ventilation Design Guidebook
P. 318
274 CHAPTER 5 PHYSIOLOGICAL AND TOXICOLOGICAL CONSIDERATIONS
The Michaelis-Menten equation is written
;
where i ;- is the measured initial velocity of an enzymatic reaction, i> m, lx is the
maximal velocity of the enzymatic reaction, and K m is the Michaelis constant.
Note that when [S] far exceeds the K m, the initial velocity, z>,-, is close to the
maximal velocity, f max.
In zero-or'W kinetics, a constant amount of a chemical compound is excreted
per unit of time. In most cases, this phenomenon is caused by the saturation of a
rate-limiting enzyme, and the enzyme commonly functions at its maximal rate,
i.e., a constant amount of a chemical compound is metabolized per unit time. A
good example is ethyl alcohol; alcohol dehydrogenase becomes saturated at rela-
tively low concentrations. Because of this saturation, ethyl alcohol is eliminated at
a constant rate about 7 g/h. However, the reason is not always an enzyme; any
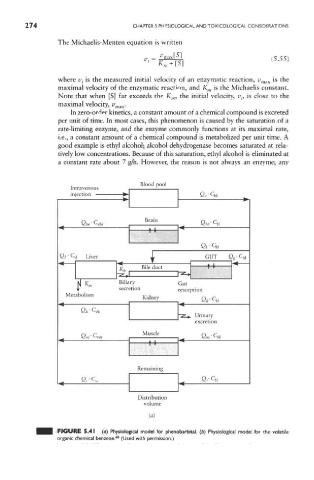
FIGURE 5.41 (a) Physiological modet for phenobarbital. (b) Physiological model for the volatile
68
organic chemical benzene. (Used with permission.)