Page 387 - Industrial Wastewater Treatment, Recycling and Reuse
P. 387
Phenolic Wastewater Treatment: Development and Applications of New Adsorbent Materials 359
is present instead of 4-CP. The equilibrium uptake showed that there is an
1
increase in adsorption capacity—reaching 96.11 mg g —while the single-
solute study demonstrated a lower uptake of 69.70 mg g 1 (Ahmaruzzaman
and Laxmi Gayatri, 2011). On the other hand, there is a decrease in the
removal of 4-NP in the binary mixture when the experimental adsorption
capacity is decreased to 98.91 mg g 1 as compared to 549.01 mg g 1
(Ahmaruzzaman and Laxmi Gayatri, 2011) in the single-solute system.
Thus, the presence of phenol in the binary solute mixture has an antagonistic
effect on the simultaneous adsorption of 4-NP, while phenol itself experi-
ences a synergistic effect. This shows the competition for the components
for the specific adsorption sites, which are readily more accessible to phenol
rather than to 4-NP due to the smaller size of the phenol molecule, and
which has greater affinity toward the micropores of ANL. A close observa-
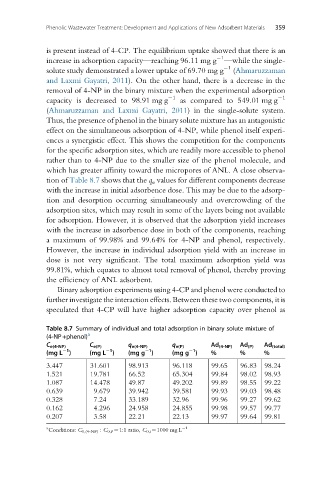
tion of Table 8.7 shows that the q e values for different components decrease
with the increase in initial adsorbence dose. This may be due to the adsorp-
tion and desorption occurring simultaneously and overcrowding of the
adsorption sites, which may result in some of the layers being not available
for adsorption. However, it is observed that the adsorption yield increases
with the increase in adsorbence dose in both of the components, reaching
a maximum of 99.98% and 99.64% for 4-NP and phenol, respectively.
However, the increase in individual adsorption yield with an increase in
dose is not very significant. The total maximum adsorption yield was
99.81%, which equates to almost total removal of phenol, thereby proving
the efficiency of ANL adsorbent.
Binary adsorption experiments using 4-CP and phenol were conducted to
further investigate the interaction effects. Between these two components, it is
speculated that 4-CP will have higher adsorption capacity over phenol as
Table 8.7 Summary of individual and total adsorption in binary solute mixture of
(4-NP+phenol) a
C e(4-NP) C e(P) q e(4-NP) q e(P) Ad (4-NP) Ad (P) Ad (total)
1
1
1
1
(mg L ) (mg L ) (mg g ) (mg g ) % % %
3.447 31.601 98.913 96.118 99.65 96.83 98.24
1.521 19.781 66.52 65.304 99.84 98.02 98.93
1.087 14.478 49.87 49.202 99.89 98.55 99.22
0.639 9.679 39.942 39.581 99.93 99.03 98.48
0.328 7.24 33.189 32.96 99.96 99.27 99.62
0.162 4.296 24.958 24.855 99.98 99.57 99.77
0.207 3.58 22.21 22.13 99.97 99.64 99.81
a 1
Conditions: C 0,(4-NP) : C 0,P ¼1:1 ratio, C 0,i ¼1000 mg L