Page 247 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 247
Isotope Dil~tion Muss Spectrometry 233
2
0.2820
18
16
c
2 14
4
+k-
12
.-
IO
8
6
0.2804
0 0.005 0.010 0.015 0.020 0.025
‘16Lu I I7?Hf
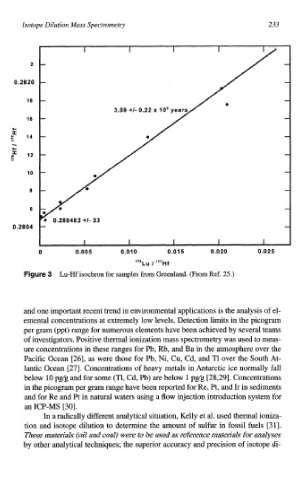
Figure 3 Lu-Hf isochron for samples from Greenland. (From Ref. 25.)
is
and one important recent trend in environmental applications the analysis of el-
emental concentrations at extremely low levels. Detection limits in the picogram
per gram (ppt) range for numerous elements have been achieved several teams
by
of investigators. Positive thermal ionization mass spectrometry was used meas-
to
ure concentrations in these ranges for Pb, Rb, and Ba in the atmosphere over the
Pacific Ocean [26], as were those for Pb, NI, Cu, Cd, and T1 over the South At-
lantic Ocean [27]. Concen~ations of heavy metals in Antarctic ice normally fall
below 10 pg‘g and for some (Tl, Cd, Pb) are below 1 pg‘g [28,29]. Concen~ations
for
in the picogram per gram range have been reported Re, Pt, and Ir in sediments
for
and for Re and Pt in natural waters using a flow injection introduction system
an ICP-MS [ 301.
et
In a radically different analytical situation, Kelly al. used thermal ioniza-
tion and isotope dilution to determine the amount of sulfur in fossil fuels E3 l].
These materials (oil and coal) were to be used as reference materials for analyses
by other analytical techniques; the superior accuracy and precision isotope di-
of