Page 242 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 242
228
rnlx
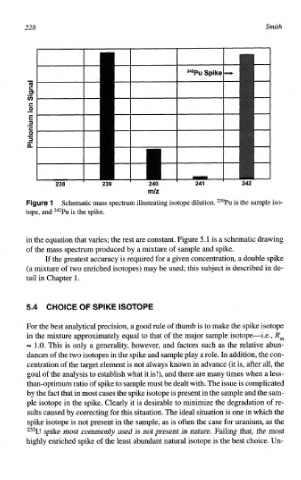
ure 1 Schematic mass spectrum illus~ating isotope dilution. 239Pu is the sample iso-
tope, and 242Pu is the spike.
in the equation that varies; the rest are constant. Figure 5.1 is a schematic drawing
of the mass spectrum produced by a mixture of sample and spike.
If the greatest accuracy is required for a given concentration, a double spike
(a mixture of two enriched isotopes) may be used; this subject is described in de-
tail in Chapter l.
For the best analytical precision, a good rule of thumb is to make the spike isotope
in the mixture approximately equal to that of the major sample isotope-i.e., R,
1.0. This is only a generality, however, and factors such as the relative abun-
dances of the two isotopes in the spike and sample play a role. In addition, the con-
centration of the target element is not always known in advance (it is, after all, the
goal of the analysis to establish what it is!), and there are many times when a less-
than-optimu~ ratio of spike to sample must be dealt with. The issue is complicated
by the fact that in most cases the spike isotope is present in the sample and the sam-
ple isotope in the spike. Clearly it is desirable to minimize the degradation of re-
sults caused by correcting for this situation. The ideal situation is one in which the
spike isotope is not present in the sample, as is often the case for uranium, as the
233U spike most commonly used is not present in nature. Failing that, the most
highly enriched spike of the least abundant natural isotope is the best choice. Un-