Page 78 - Inorganic Mass Spectrometry : Fundamentals and Applications
P. 78
Olesik
transform ion cyclotron resonance mass spectrometers has also been reported.
Although most samples are introduced into the instrument as solutions, laser
ablation and other solid sampling approaches are also available comercially.
Although ICP-MS is a highly successful and powerful technique, several
problems remain. When attempting to measure low concentrations (sub-part per
billion) spectral overlaps due to polyatomic ions can be difficult to identify and
overcome, particularly with quadrupole or time-of-~ight mass spectrometers.
Iron, calcium, and potassium typically suffer from rather severe spectral overlaps
unless special steps are taken. Arsenic, selenium, c~o~urn, vanadium, and
titanium often suffer from polyatomic ion spectral overlaps that degrade detection
limits. Molecular ion spectral overlaps are most common below mass 82 [33.
Matrix effects due to high concentrations of concomitant species, particularly
heavy elements, can be severe when conditions are optimized for ~~imum
analyte sensitivity. Because the sample must be physically transported into the
mass spectrometer, deposition and contamination of the ins~ment can be prob-
lems (unlike ICP optical emission spectroscopy, in which photons are clean). For
some applications short- and long-term precision is inadequate. In some applica-
tions, even better detection limits or sensitivities are required. The initial equip-
ment and operating costs are high.
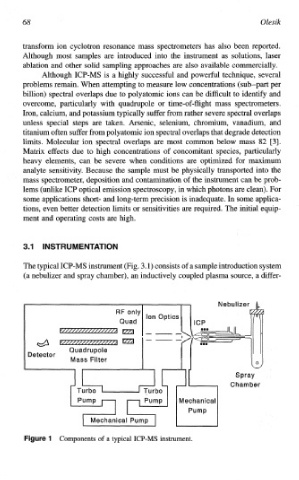
3.1) consists of a sample introduction system
The typical ICP-MS instrument (Fig.
(a nebulizer and spray chamber), an inductively coupled plasma source, a differ-
1 Components of a typical ICP-MS instrument.