Page 118 - Instant notes
P. 118
Physical chemistry 104
increasing the pressure produces a smaller increase in the boiling point than the melting
point.
If dp/dT is changed according to this equation, thereby ensuring that equilibrium is
maintained, the increase in pressure tends to compress the vapor volume, increasing its
density, whilst the increase in temperature tends to weaken the liquid intermolecular
forces, decreasing its density. Eventually, at the critical point, characterized by a critical
pressure and a critical temperature, the densities of vapor and liquid become equal, the
two phases are indistinguishable and there is no longer any measurable phase transition.
Phase diagrams of a single species
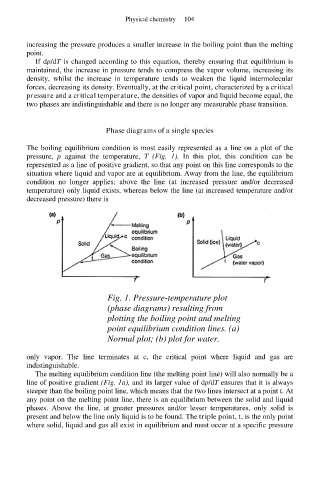
The boiling equilibrium condition is most easily represented as a line on a plot of the
pressure, p against the temperature, T (Fig. 1). In this plot, this condition can be
represented as a line of positive gradient, so that any point on this line corresponds to the
situation where liquid and vapor are at equilibrium. Away from the line, the equilibrium
condition no longer applies; above the line (at increased pressure and/or decreased
temperature) only liquid exists, whereas below the line (at increased temperature and/or
decreased pressure) there is
Fig. 1. Pressure-temperature plot
(phase diagrams) resulting from
plotting the boiling point and melting
point equilibrium condition lines. (a)
Normal plot; (b) plot for water.
only vapor. The line terminates at c, the critical point where liquid and gas are
indistinguishable.
The melting equilibrium condition line (the melting point line) will also normally be a
line of positive gradient (Fig. 1a), and its larger value of dp/dT ensures that it is always
steeper than the boiling point line, which means that the two lines intersect at a point t. At
any point on the melting point line, there is an equilibrium between the solid and liquid
phases. Above the line, at greater pressures and/or lesser temperatures, only solid is
present and below the line only liquid is to be found. The triple point, t, is the only point
where solid, liquid and gas all exist in equilibrium and must occur at a specific pressure