Page 136 - Instant notes
P. 136
Physical chemistry 122
For salt dissolution to be a spontaneous process, the change in Gibbs free energy,
∆G (see Topic B6) of the dissolution process must be negative. Thus, endothermic salt
dissolution is a spontaneous process if T∆S is sufficiently positive (see Topic B6, Table
1).
Qualitative treatment of ionic interaction
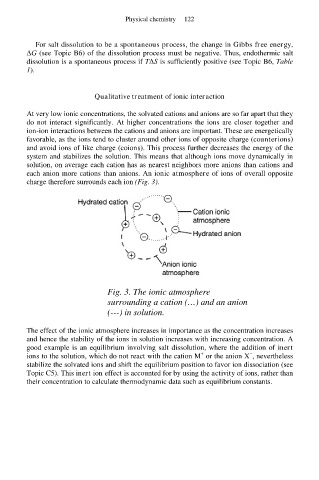
At very low ionic concentrations, the solvated cations and anions are so far apart that they
do not interact significantly. At higher concentrations the ions are closer together and
ion-ion interactions between the cations and anions are important. These are energetically
favorable, as the ions tend to cluster around other ions of opposite charge (counterions)
and avoid ions of like charge (coions). This process further decreases the energy of the
system and stabilizes the solution. This means that although ions move dynamically in
solution, on average each cation has as nearest neighbors more anions than cations and
each anion more cations than anions. An ionic atmosphere of ions of overall opposite
charge therefore surrounds each ion (Fig. 3).
Fig. 3. The ionic atmosphere
surrounding a cation (…) and an anion
(---) in solution.
The effect of the ionic atmosphere increases in importance as the concentration increases
and hence the stability of the ions in solution increases with increasing concentration. A
good example is an equilibrium involving salt dissolution, where the addition of inert
+
−
ions to the solution, which do not react with the cation M or the anion X , nevertheless
stabilize the solvated ions and shift the equilibrium position to favor ion dissociation (see
Topic C5). This inert ion effect is accounted for by using the activity of ions, rather than
their concentration to calculate thermodynamic data such as equilibrium constants.