Page 134 - Instant notes
P. 134
Physical chemistry 120
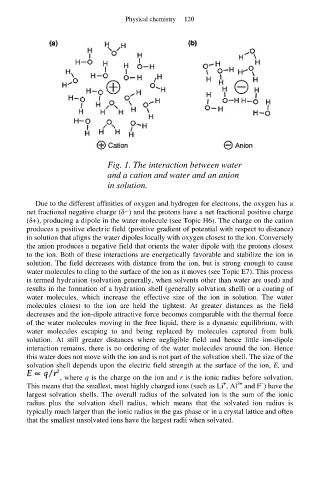
Fig. 1. The interaction between water
and a cation and water and an anion
in solution.
Due to the different affinities of oxygen and hydrogen for electrons, the oxygen has a
net fractional negative charge (δ−) and the protons have a net fractional positive charge
(δ+), producing a dipole in the water molecule (see Topic H6). The charge on the cation
produces a positive electric field (positive gradient of potential with respect to distance)
in solution that aligns the water dipoles locally with oxygen closest to the ion. Conversely
the anion produces a negative field that orients the water dipole with the protons closest
to the ion. Both of these interactions are energetically favorable and stabilize the ion in
solution. The field decreases with distance from the ion, but is strong enough to cause
water molecules to cling to the surface of the ion as it moves (see Topic E7). This process
is termed hydration (solvation generally, when solvents other than water are used) and
results in the formation of a hydration shell (generally solvation shell) or a coating of
water molecules, which increase the effective size of the ion in solution. The water
molecules closest to the ion are held the tightest. At greater distances as the field
decreases and the ion-dipole attractive force becomes comparable with the thermal force
of the water molecules moving in the free liquid, there is a dynamic equilibrium, with
water molecules escaping to and being replaced by molecules captured from bulk
solution. At still greater distances where negligible field and hence little ion-dipole
interaction remains, there is no ordering of the water molecules around the ion. Hence
this water does not move with the ion and is not part of the solvation shell. The size of the
solvation shell depends upon the electric field strength at the surface of the ion, E, and
, where q is the charge on the ion and r is the ionic radius before solvation.
−
3+
+
This means that the smallest, most highly charged ions (such as Li , Al and F ) have the
largest solvation shells. The overall radius of the solvated ion is the sum of the ionic
radius plus the solvation shell radius, which means that the solvated ion radius is
typically much larger than the ionic radius in the gas phase or in a crystal lattice and often
that the smallest unsolvated ions have the largest radii when solvated.